Search API
The Florida Department of Health published Arbovirus Surveillance Update #15, which discloses various mosquito-borne diseases reported this year.
As of April 13, 2024, countries in southern Florida confirmed these mosquito-transmitted diseases:
- Chikungunya - Three cases of chikungunya that began in 2024 have been reported in individuals with a travel history to Brazil, and four cases were confirmed in 2023.
- Dengue Fever - In 2024, 106 travel-associated dengue cases have been reported, mainly by visitors from Cuba. In 2023, 609 travel-associated dengue cases were reported, primarily in people arriving from Brazil and Cuba. Five cases of locally acquired dengue were reported from Miami-Dade (4) and Pasco counties in 2024.
- Malaria - In 2024, nineteen cases of malaria with onset in 2024 have been reported in individuals with a travel history to malaria-endemic areas in Africa. In 2023, 78 malaria cases were reported.
To learn where these diseases are spreading, the U.S. CDC issued Travel Health Notices to inform travelers about global health risks.
While the U.S. FDA recently approved an innovative chikungunya vaccine, its availability in Florida is pending. Access to the approved dengue vaccine has specific requirements.
Furthermore, two approved malaria vaccines are unavailable in the U.S.
Visit a local travel clinic, such as Passport Health USA Tampa, to learn more about these and other travel vaccine options.

Moderna, Inc. today announced a contract with Brazil's Ministry of Health (Ministério da Saúde) to supply its monovalent mRNA COVID-19 vaccine.
Under the new contract, 12.5 million doses of Moderna's mRNA COVID-19 vaccine (SpikeVax) are anticipated for delivery in the second quarter of 2024.
This contract follows the Brazilian Health Regulatory Agency's approval of Moderna's COVID-19 vaccine (XBB.1.5 sublineage) in March 2024 to prevent COVID-19 in children from six months of age and adults.
"We are proud to partner with the Ministry of Health to provide our mRNA COVID-19 vaccine for the first time in Brazil as part of the national vaccination campaign," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release on April 22, 2024.
"This agreement underscores our commitment to global health and our role in supporting Brazil's efforts to protect its citizens against COVID-19."
"We look forward to working with the Ministry of Health to help maintain health security in Brazil."
Previously, Moderna generated preclinical and clinical data of its monovalent XBB.1.5 vaccine candidate, which showed an immune response against XBB sublineages and currently circulating strains of the SARS-CoV-2 virus, including JN.1.

Although antiretroviral treatment (ART) can help manage the impact of the Human Immunodeficiency Virus (HIV), it is not a cure. People living with HIV need to take the treatment for their entire lives to suppress viral replication and protect their immune systems.
To address this clinical need, ACTG, a global clinical trials network focused on HIV and other infectious diseases, today announced the opening of A5374, a phase 1/2a study evaluating the safety, tolerability, and antiviral effect of a novel combination regimen that includes therapeutic T-cell vaccines, two broadly neutralizing antibodies (3BNC117-LS, 10-1074-LS), and an immune-system boosting treatment among people living with HIV who started ART during acute HIV infection.
This study hypothesizes that this combination regimen will be safe and result in a more extended period of viral control during a closely monitored pause in ART (known as an analytic treatment interruption) compared to placebo.
The approach being studied in A5374 is part of a potential path toward enabling the immune system to manage HIV in the absence of ART for weeks or months.
“We expect that controlling HIV in the absence of ART will require a combination of novel treatments to decrease the number of cells harboring HIV and simultaneously stimulate the immune system to keep the virus from replicating,” said ACTG Chair Judith Currier, M.D., M.Sc., University of California Los Angeles in a press release.
“A5374 is a pivotal trial, and we hope it will provide us with important insights into how to boost the immune system to control HIV.”
ACTG says carefully monitored analytic treatment interruptions are an essential part of HIV cure clinical trials. They can help determine whether a research intervention can reduce latent HIV (HIV that is “hidden” in different cells and tissues but not replicating) or increase immune function and delay or prevent latent HIV from actively replicating.
While there are no U.S. FDA-approved HIV prevention vaccines today, clinical development accelerated in 2023, with vaccine candidates utilizing innovative technologies such as mRNA.

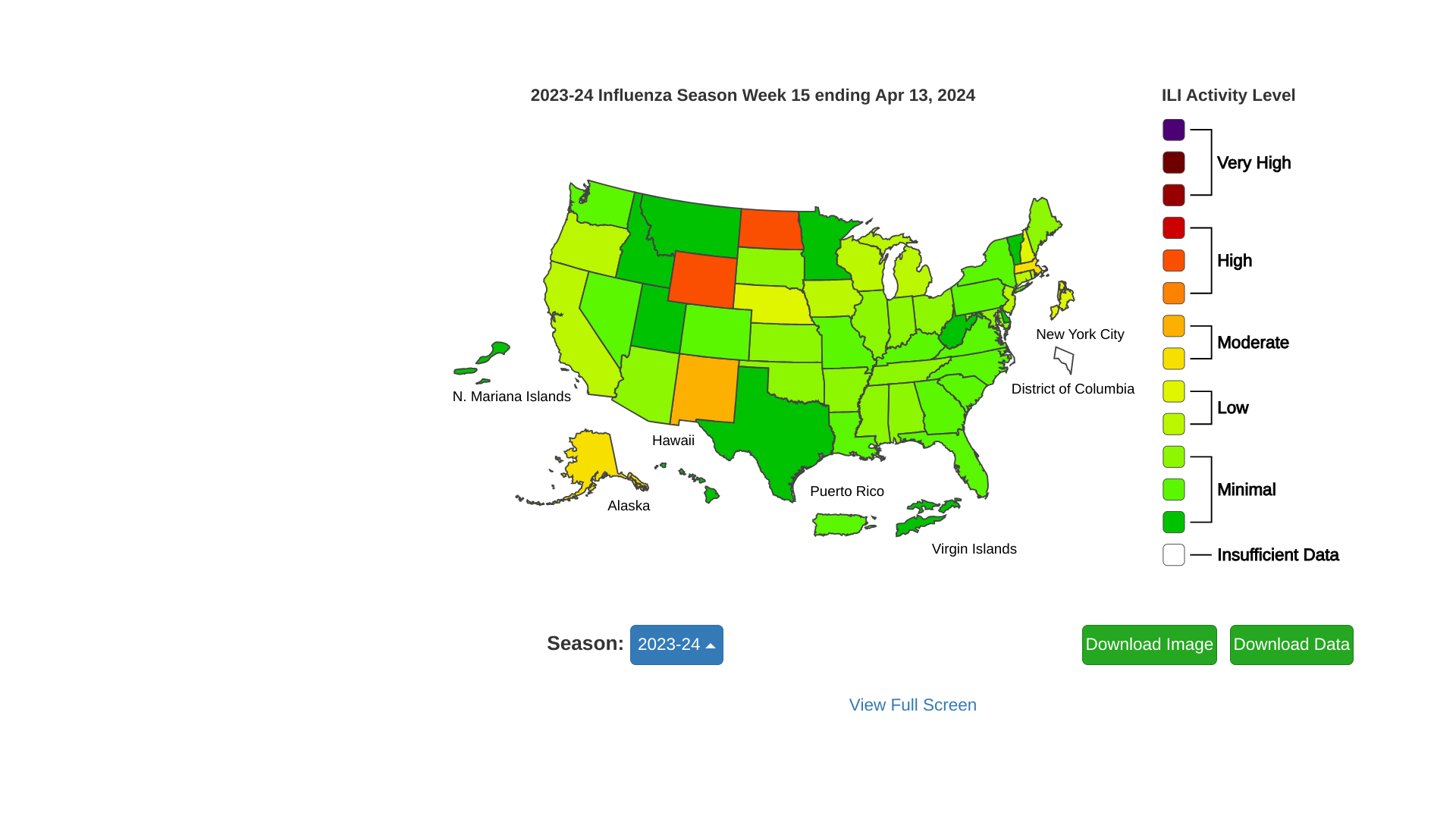
The U.S. CDC FluView report for week #15 stated seasonal influenza activity continues to decline in most areas of the United States.
Nationally, the number of weekly flu hospital admissions has been decreasing since January 2024.
Unfortunately, four influenza-associated pediatric deaths occurring during the 2023-2024 season were reported to the CDC last week, bringing the flu season total to 142 pediatric deaths.
As of April 19, 2024, the CDC recommends that everyone six months and older get an annual flu vaccine as long as influenza viruses spread.
Flu shots (egg, cell, nasal-based) can still provide benefits this season.
According to the recent Nationally Notifiable Infectious Diseases and Conditions weekly report, the number of mpox cases in the United States has more than doubled compared to Week #15 in 2023.
As of April 13, 2024, 750 mpox cases had been reported, compared to 336 cases at the same time last year.
The U.S. CDC highlights New York City (151), California (72), and Texas (55) as mpox case leaders.
The U.S. Health and Human Services (HHS) was initially charged with coordinating the federal response to the mpox outbreak. According to the General Accountability report issued on April 18, 2024, HHS is recommended to adopt a coordinated, department-wide program that incorporates input from external stakeholders to identify and resolve challenges.
In the United States, Bavarian Nordic's JYNNEOS® vaccine was initially offered to healthcare staff in Boston on May 24, 2022.
Since then, over 1.2 million (1-dose: 38.8% and 2-dose: 24.3%) JYNNEOS doses have been administered in U.S. Jurisdictions.
As of April 2024, JYNNEOS remains the only FDA-approved non-replicating smallpox and mpox vaccine for military and non-military use and has recently become commercially available at U.S. pharmacies.

The WHO Director-General convened the thirty-eighth meeting of the Emergency Committee on the international spread of poliovirus in late March 2024.
The Committee unanimously agreed that the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern and recommended the extension of Temporary Recommendations for a further three months until July 2024.
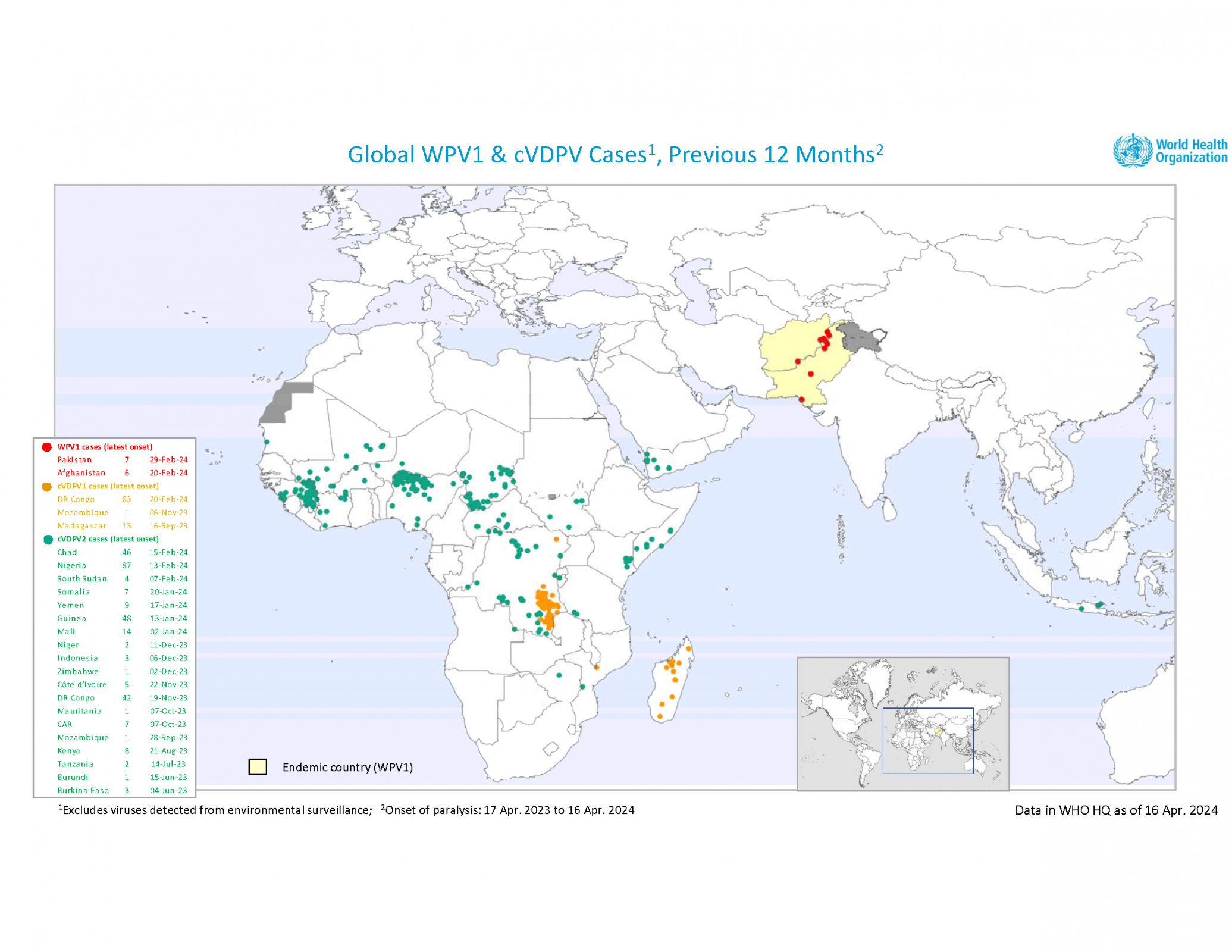
Regarding a weekly update, the Global Polio Eradication Initiative reported on April 17, 2024, that three African countries reported wild poliovirus (WPV1) and circulating vaccine-derived polioviruses (cVDPV) cases last week.
Chad reported one cVDPV2 case in Mandoul, making it the first in 2024. The number of 2023 cases remains 55.
The Democratic Republic of the Congo reported one cVDPV1 case in Haut-Katanga, the first this year. The number of polio cases in 2023 remains at 106, and 117 cases of cVDPV2 in 2023.
Nigeria confirmed one cVDPV2 case last week in Kebbi, the eighth this year. There have been 87 cases reported in 2023.
The U.S. CDC publishes travel advisories for countries reporting active polio cases and recommends fully vaccinating people before visiting these countries.
To understand polio vaccination options, including booster doses, the CDC suggests speaking with a travel vaccine advisor one month before traveling abroad.
Various media sources reported Merck would only supply 18.8 million HPV vaccine doses to Gavi-supported countries in 2024. Merck had previously promised to supply 29.6 million doses.
According to the Peoples Gazette, on April 19, 2024, Merck spokesman Patrick Ryan said the company "experienced a manufacturing disruption," requiring it to hold and check numerous doses manually.
Ryan disclosed that Burundi, Tajikistan, Mozambique, Sierra Leone, Ivory Coast, and Burkina Faso will not receive HPV vaccines in 2024.
By the end of 2022, 32 countries had successfully launched their HPV vaccine national program with Gavi support, fully immunizing more than 16.3 million girls since 2014.
GAVI says HPV vaccination is critical to reducing cervical cancer, especially in lower-income countries with a high disease burden and less developed cervical cancer screening and treatment programs.
On March 13, 2024, Merck announced plans to initiate clinical development of a new investigational multi-valent HPV vaccine designed to provide broader protection against multiple HPV types.
Separately, the company also plans to conduct clinical trials in both females and males to evaluate the efficacy and safety of a single-dose regimen of the GARDASIL®9 vaccine compared to the approved three-dose regimen.
"Evidence continues to emerge showing the importance of GARDASIL and GARDASIL 9 to public health," said Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, in a press release.
"These significant investments build upon our leadership and, importantly, provide the opportunity to further impact the global burden of certain HPV-related cancers and diseases."
The latest addition to Merck's pipeline employs the company's proprietary virus-like particle (VLP) technology to incorporate additional VLPs for expanded HPV-type coverage.
This includes several types known to have a greater impact on African and Asian populations and individuals of African and Asian descent. First-in-human phase 1 clinical studies are scheduled to start in the fourth quarter of 2024.
In the U.S., HPV vaccines remain available at clinics and pharmacies in 2024.