Search API
Recce Pharmaceuticals Ltd. today announced an Independent Safety Committee approved an increase in dosing to 4,000mg over a fast infusion of 30 minutes in a Phase I/II clinical trial evaluating its lead candidate, RECCE® 327 (R327).
The Company says it identified 30 minutes as the optimum infusion time and increased to a higher concentration per regulatory expectations to investigate R327’s high concentration potential.
“We’re thrilled the independent safety committee has unanimously clearly an increased R327 dose to 4,000mg, over a 30-minute fast IV infusion,” said James Graham, Chief Executive Officer of Recce Pharmaceuticals, in a press release on April 30, 2024.
“The ability to administer high concentrations of a broad-spectrum anti-infective underscores the potential of a novel treatment for millions of patients worldwide with urinary tract infections (UTI) or urosepsis each year.”
Dosing has successfully achieved Minimum Inhibitory Concentration activity among existing clinical samples. The Company has now dosed 3,000mg at multiple infusion times: 15, 20, 30, 45 minutes, and 1 hour.
In accordance with the study protocol, the efficacy of R327 via IV administration will be made available at the completion of the ACTRN12623000448640 trial.
Recce’s New Class of Synthetic Anti-Infectives have a universal mechanism of action with the ability to overcome hyper-cellular mutation of bacteria and viruses. RECCE® 327 is not a preventive vaccine and is not approved for use in humans in any country.
The investigational recurrent UTI vaccine MV140 is available in Australia and various countries in 2024.

Although there is no approved vaccine to prevent norovirus, it is estimated that approximately 21 million people in the United States are infected with this virus every year, including about 15% of children under the age of 5.
While pediatric deaths resulting from norovirus are rare in the U.S., they are more common in developing countries.
Nevertheless, the economic burden of norovirus in the United States alone is estimated to be $10.6 billion annually.
A recently completed Phase I trial, partially funded by the Bill & Melinda Gates Foundation, included immunogenicity measures.
On April 30, 2024, Vaxart, Inc. announced that it had completed the topline analysis evaluating the Company's orally administered bivalent GI.1/GII.4 norovirus vaccine candidate.
The VXA-NVV-108 clinical trial focused on vaccinating lactating mothers.
Antibodies to norovirus rose on average four-fold for the G1.1 virus strain and six-fold for the GII.4 virus strain in the breast milk of lactating mothers who received the Vaxart vaccine candidate in the high dose group.
Furthermore, there were no vaccine-related serious adverse events, no dose-limiting pharmacotoxicity, and no new onset of chronic illness was observed through the active period.
"This is an important step forward as we drive toward a vaccine candidate that may make it possible for mothers to protect their children against this highly contagious – and potentially lethal – virus."
"It can be difficult to immunize the youngest children mucosally because the immune system is still developing."
"Passive transfer of antibodies from mothers to infants via breast milk is an innovative approach to potentially improve infection resistance in infants," said Dr. James F. Cummings, Vaxart's Chief Medical Officer, in a press release.
As of late April 2024, the World Health Organization has not pre-qualified any norovirus vaccine candidate.

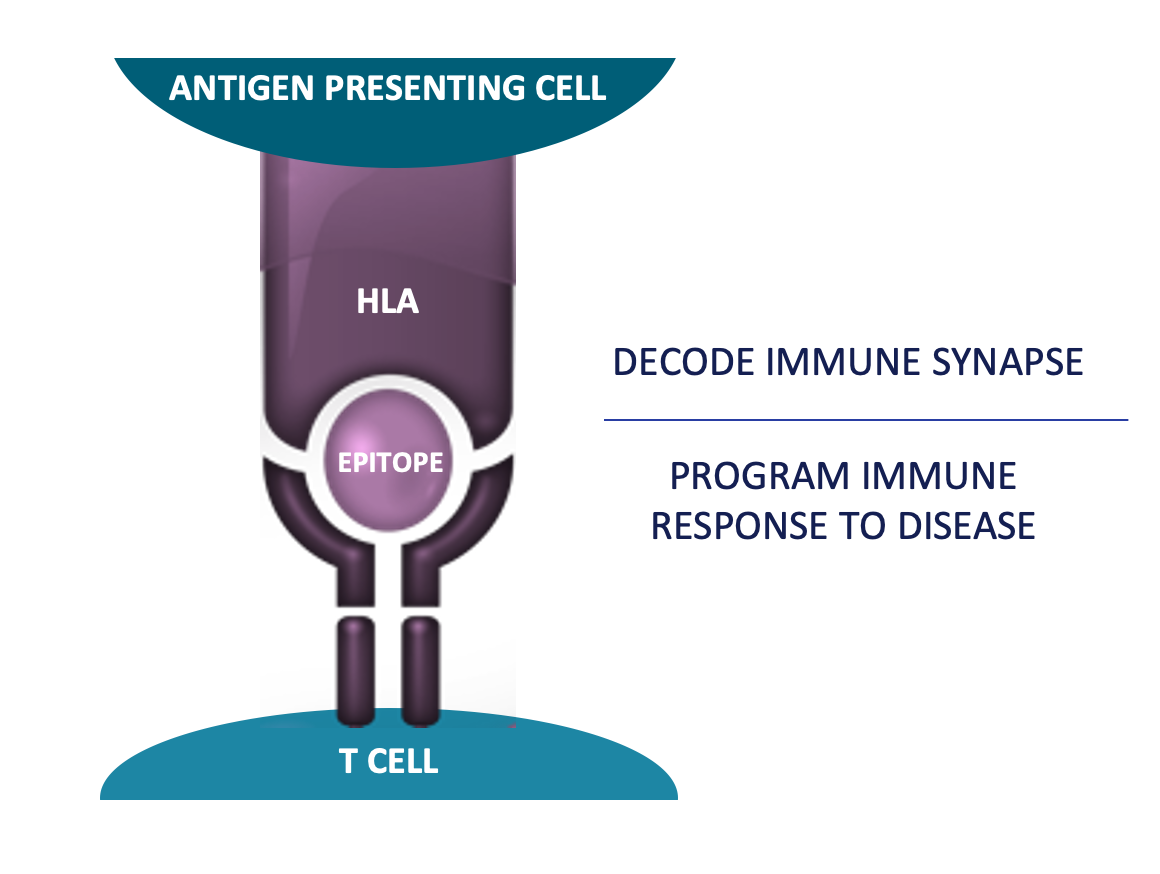
Repertoire® Immune Medicines announced today that it has entered a multi-year strategic collaboration with Bristol Myers Squibb to develop tolerizing vaccines for up to three autoimmune diseases.
Repertoire is developing tolerizing vaccines as a new class of programmable medicines for patients with autoimmune diseases.
These vaccines have the potential to re-establish immune homeostasis, leading to durable disease remission in the absence of generalized immune suppression, thereby overcoming the limitations of today's medicines and transforming the lives of patients who have been diagnosed with an autoimmune disease.
The collaboration aims to develop efficacious, selective, and durable treatments for patients suffering from autoimmune disease by resetting the immune system.
Repertoire will lead all activities through to development candidate nomination, while BMS will lead clinical development, regulatory affairs, and commercialization of the tolerizing vaccines under an exclusive worldwide license.
Repertoire will utilize its T cell receptor (TCR)-epitope discovery platform, DECODE, and its proprietary lipid nanoparticle delivery technology to discover and develop the tolerizing vaccine development candidates.
In addition, it will deploy DECODE to monitor immune responses to the tolerizing vaccines in patients during clinical development to provide key insights into the pharmacodynamic effect of the vaccines.
"This agreement is a recognition of the transformative power of Repertoire's DECODE platform to discover and develop programmable T cell targeted immune medicines," said Torben Straight Nissen, Ph.D., CEO of Repertoire and Executive Partner of Flagship Pioneering, in a press release on April 29, 2024.
"We are excited to collaborate with Bristol Myers Squibb to combine their leadership in immunology with our unique ability to discover key disease-associated epitopes in patients with autoimmune diseases. This collaboration enables us to serve patients suffering from autoimmune diseases by translating our DECODE discoveries into potentially transformative medicines that address the underlying cause of their disease."
Under the terms of the agreement, Repertoire will receive an upfront payment of $65 million and up to $1.8 billion for achieving development, regulatory, and commercial milestones in addition to receiving tiered royalties.
Merck today announced results from STRIDE-10, a Phase 3 trial evaluating V116, the company’s investigational, adult-specific 21-valent pneumococcal conjugate vaccine.
Key results from the study include:
V116 elicited immune responses that were noninferior compared to PPSV23 for the 12 serotypes (or strains) common to both vaccines, as measured by serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) at Day 30.
Immune responses elicited by V116 were superior for the nine serotypes included in V116 but not PPSV23, as measured by OPA GMT ratios at Day 30, and superior for eight of nine serotypes unique to V116 compared to PPSV23, as measured by the proportions of participants with ≥4-fold rise in immune responses.
V116 had a safety profile comparable to PPSV23.
“Invasive pneumococcal disease and pneumococcal pneumonia represent significant public health challenges, particularly among older adult populations and those with risk conditions,” said Dr. Walter Orenstein, professor emeritus of medicine, epidemiology, global health and pediatrics at Emory University and member of Merck’s Scientific Advisory Committee, in a press release on April 29, 2024.
“These positive results show that V116 has the potential to help prevent invasive pneumococcal disease among adult populations.”
In addition to the clinical data on V116, Merck also presented findings that suggest V116 may help to reduce the health and economic burden associated with invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia among adults in France, Sweden, Spain, and the Netherlands.

With the creation of efficacious cancer prevention vaccines to target human papillomavirus (HPV) in the first decade of this century, the World Health Organization (WHO) set an ambitious target to lower cervical cancer incidence and mortality by 30% by 2030.
While the WHO targets are aspirational, no country has yet verified that it has reached them.
A presentation by Professor Suzanne Garland at this year's ESCMID Global Congress revealed that even though the evidence is clear and continues to build that HPV vaccination is reducing cervical cancer incidence and mortality and HPV-related disease, there are high variations in coverage globally.
Prof Garland reviewed the WHO Dashboard data, which shows that of 194 reporting countries, 137 (71%) have HPV in their national vaccination programs.
The WHO database shows that the average full vaccine coverage is 44% globally.
Specifically, it shows that Canada, Ireland, Sweden, Spain, and Portugal have full vaccination coverage above 70%, while the USA and Germany trail behind at 50-70%.
Of the HPV national programs reporting to the end of 2023, 42% are for both sexes, while 58% are for girls only.
Globally, 21% of girls have received at least one dose of HPV vaccine by age 15, which has steadily increased from 4% in 2010.
Prof Garland concluded in a press release on April 27, 2024, "Vaccination is a critical component of the global strategy to eliminate cervical cancer as a public health problem."
"There is strong and growing evidence on effectiveness against cervical cancer, with rates falling steadily as vaccination takes effect."
"Scaling up vaccine access and coverage globally is critical to reduce inequities between and within countries."
In the United States, several states are reaching a broad number of boys and girls, while others are lagging.
For example, Texas's HPV vaccination rate for children ages 13–17 is below the national average, ranking 48th out of 50 states and the District of Columbia in 2021.
In 2021, 51.5% of Texas teens completed the HPV vaccine series.
As of April 29, 2024, HPV vaccines are available at clinics and pharmacies in the U.S.

Research findings presented at the European Society of Clinical Microbiology and Infectious Diseases Global Congress show that a lower dose of the JYNNEOS® (MVA-BN) mpox vaccine is safe and generates a six-week antibody response equivalent to the standard regimen.
The results announced by the U.S. NIH on April 27, 2024, suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
The authors noted that because no defined correlates of protection against mpox—immune processes confirmed to prevent disease—these findings cannot predict the efficacy of dose-sparing regimens with certainty.
Real-world data from the U.S. Centers for Disease Control and Prevention and others have shown similar vaccine effectiveness for the dose-sparing regimen given intradermally and the standard regimen given subcutaneously.
According to the NIH, a study of the standard JYNNEOS regimen in adolescents is ongoing and will report findings later this year.
An earlier press release stated that the antibodies produced by JYNNEOS against mpox wane significantly within a year of receiving the vaccination. In contrast, among individuals who had received childhood smallpox vaccination, most had detectable VACV IgG one year after vaccinations.
In April 20224, JYNNEOS became commercially available in the U.S. by establishing additional pathways for vaccine procurement, distribution, and reimbursement by public and private payers, including community pharmacies.
Mpox vaccinations are essential since the number of mpox cases in the U.S. has more than doubled compared to Week #15 in 2023. As of April 13, 2024, 750 mpox cases had been reported, compared to 336 cases at the same time last year.