Search API
BioNTech SE today reported financial results for the first three months of 2024, and provided an update on its corporate progress.
Total revenues reported were €187.6 million for the three months in 2024, compared to €1,277 million for the comparative period in 2023.
The Germany-based company stated the year-over-year change was mainly due to lower commercial revenues from the sales of BioNTech’s COVID-19 vaccine worldwide, resulting from endemic-level demand for COVID-19 vaccines.
“In the past weeks, we have reported positive preliminary data for our individualized and off-the-shelf mRNA-based candidates, which further underline the potential of our iNeST and FixVac platforms. We look forward to providing more updates this year across our oncology portfolio, including our bispecific antibody and ADC programs,” said Prof. Ugur Sahin, M.D., CEO and Co-Founder of BioNTech, in a press release on May 6, 2024.
“In the remainder of the year, we plan to develop and commercialize a variant-adapted COVID-19 vaccine and accelerate our clinical development activities towards realizing the full potential of our oncology pipeline with a view to becoming a commercial company with marketed medicines for cancer and infectious diseases.”
BioNTech and Pfizer developed, manufactured, and delivered their Omicron XBB.1.5-adapted monovalent Comirnaty COVID-19 vaccine, which has received multiple regulatory approvals, including full approvals, authorizations for emergency or temporary use, or marketing authorizations, in more than 40 countries and regions.
BioNTech says it is now focused on preparing for variant strain vaccine adaptation to be ready for commercial launch ahead of the upcoming 2024/2025 vaccination season, pending approvals.

As outbreaks of vaccine-preventable diseases continue to occur in European countries, vaccinations and booster doses are recommended. Everyone should check with their healthcare providers to ensure they are up-to-date with recommended vaccines, says the European Centre for Disease Prevention and Control (ECDC).
Recently, the ECDC reported that between March 2023 and the end of February 2024, at least 5,770 measles cases, including at least five deaths, had been reported in the EU/EEA.
The European Union is a political and economic union of 27 European countries, while the European Economic Area is made up of 30 countries, including the EU, Iceland, Liechtenstein, and Norway.
As of 2024, the ECDC says 70% of measles outbreaks have occurred in those under ten, and 78% were contracted outside their countries or due to imported cases.
To inform international travelers, the U.S. Centers for Disease Control and Prevention recently listed the top ten international measles outbreaks led by Kazakhstan (27,280), Azerbaijan, Iraq, and India.
In the U.S., the spike in measles cases this year was primarily reported in Chicago, Illinois.

It has been about three decades since the U.S. Food and Drug Administration approved the first monoclonal antibody. Since then, antibody engineering has dramatically evolved.
The recent pandemic was the first time monoclonal antibody-based therapies were produced in significant quantities to combat a new infectious disease. Globally, clinics administered hundreds of thousands of antibody injections over the first two years of the pandemic.
Antibody therapy worked... until it didn't.
The U.S. CDC says the infectious virus's rapid evolution outpaced the benefits derived from antibodies.
According to Michael Dumiak's article published by IAVI on April 25, 2024, this experience and other issues have researchers assessing the future of antibody therapies for treating or preventing infectious diseases, including some of the most complicated pathogens, such as HIV and antibiotic-resistant bacteria.
A potential application is blocking mother-to-child transmission of HIV during birth and through the breastfeeding period.
"We are in the position that if you want more antibodies for infectious disease, you need to be very cautious," says Rino Rappuoli, scientific director of the Biotecnopolo di Siena Foundation in Italy.
The unedited, complete IAVI article is posted at this link.
Note: As of May 5, 2024, the U.S. FDA has not approved an HIV vaccine candidate.

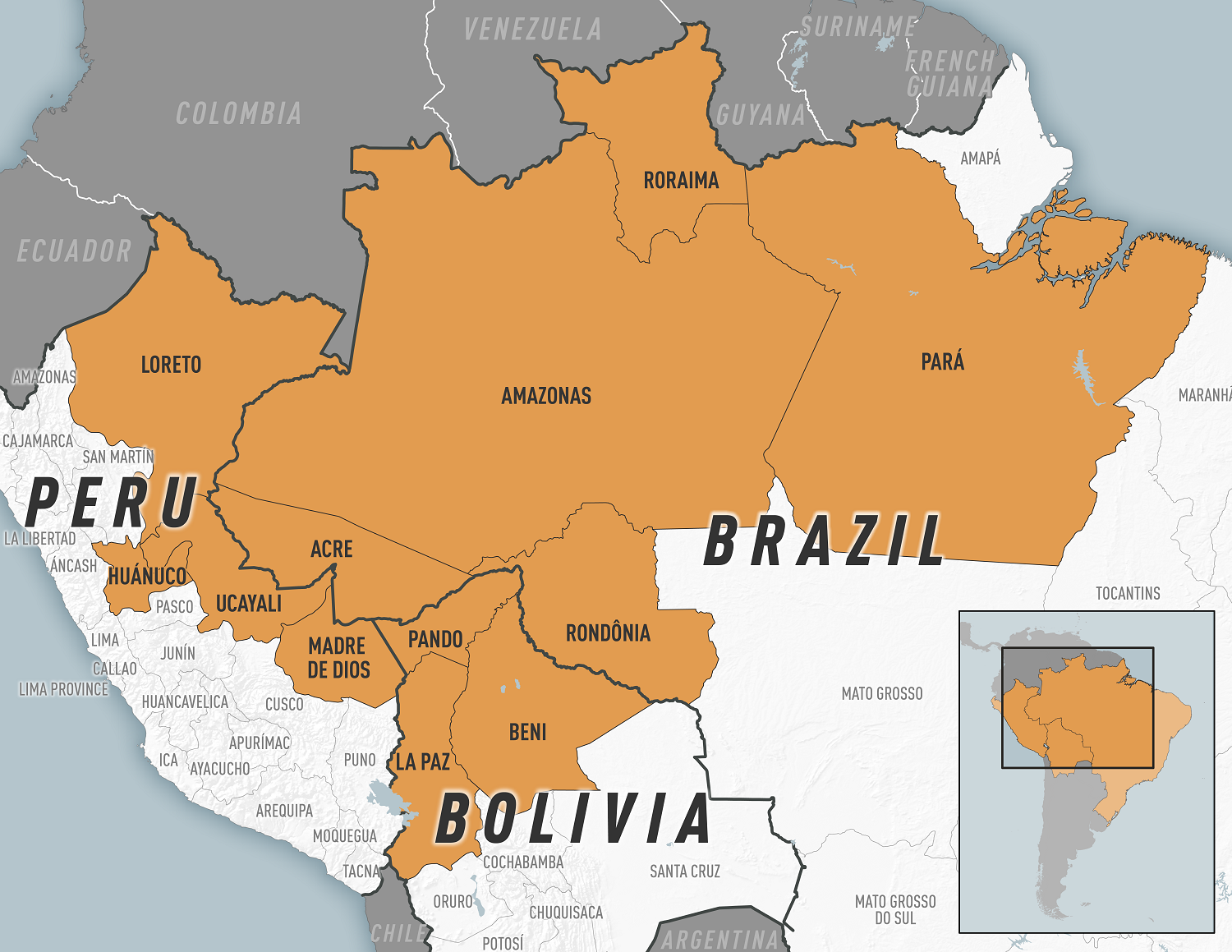
In 2024, there has been an increase in the detection of Oropouche fever outbreaks in areas of the Region of the Americas.
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed Oropouche fever outbreaks in parts of Brazil, Bolivia, and Peru.
For example, between 2023 and early 2024, 1,066 human cases of the Oropouche virus were registered in the Brazilian state of Amazonas.
To alert international travelers to this health risk, the CDC issued a Level 1, Practice Usual Precautions, Travel Health Advisory, saying Oropouche fever is spread through the bites of infected midges (flies) and Culicoides paraensis mosquitoes.
The illness is often mistaken for dengue.
The Pan American Health Organization (PAHO) says travelers to these areas should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
Most people recover without long-term effects.
Symptoms typically start 4–8 days after being bitten and last 3–6 days.
Oropouche fever has no cure or specific therapy, so treatment is symptomatic. Oral analgesics and anti-inflammatory agents can help with headaches and body pains.
As of May 2024, no licensed vaccines or specific antiviral treatments for Oropouche fever exist. However, recent clinical studies have used peptide vaccines to develop epitope-based vaccines, which could lead to potential use in the future.
New research presented at the Pediatric Academic Societies confirms rotavirus vaccines do not cause significant outbreaks of the disease in neonatal intensive care units (NICUs).
These researchers concluded that vaccine-strain rotavirus transmission in the NICU was rare and without clinical consequences.
The study found that 99.3% of non-vaccinated patients exposed to vaccinated patients did not test positive for the disease. Non-vaccinated patients who contracted rotavirus had no symptoms after 14 days.
Announced on May 3, 2024, these findings are significant because many NICUs avoid vaccinating against rotavirus due to a theoretical risk of transmission, yet some infants are too old to receive the vaccine once discharged from the NICU.
According to researchers, preterm infants are at higher risk of the highly contagious but preventable virus, yet few receive the vaccine in hospital settings.
This concern is because the rotavirus vaccine contains a weakened form of the virus.
“Immunization with rotavirus vaccine has been standard practice in the Children’s Hospital of Philadelphia NICU since 2007, and the safety of this practice was supported by retrospective clinical data published in Pediatrics in 2014 – however, this remains an uncommon practice in NICUs across the United States,” said Kathleen Gibbs, MD, the study’s lead neonatologist from Children’s Hospital of Philadelphia, in a press release.
“Our yearlong, prospective study, done in collaboration with the U.S. Centers for Disease Control and Prevention, suggests that the benefits of vaccinating NICU patients against rotavirus outweigh the risks. Inpatient vaccination allows protection of a vulnerable population against a common, preventable cause of severe diarrheal illness.”

According to researchers at the University of Minnesota, a common diabetes drug has been found to reduce the amount of SARS-CoV-2 coronavirus and lower the risk of COVID-19 rebound symptoms when administered early during non-severe illness.
"The results of the study are important because COVID-19 continues to cause illness, both during acute infection and for months after infection," said Carolyn Bramante, MD, principal investigator and an assistant professor at the U of M Medical School, in a press release on May 2, 2024.
This study was published in the journal Clinical Infectious Diseases on May 1, 2024.
These researchers wrote that in this randomized, placebo-controlled phase 3 clinical trial of outpatient treatment of SARS-CoV-2, metformin significantly reduced the viral load.
The mean SARS-CoV-2 viral load was reduced 3.6-fold with metformin relative to placebo (−0.56 log10 copies/mL; 95% confidence interval [CI], −1.05 to −.06; P = .027).
Those who received metformin were less likely to have a detectable viral load than placebo at day five or day 10 (odds ratio [OR], 0.72; 95% CI, .55 to .94).
Viral rebound, defined as a higher viral load on day ten than on day 5, was less frequent with metformin (3.28%) than with placebo (5.95%; OR, 0.68; 95% CI, .36 to 1.29).
The metformin effect was consistent across subgroups and increased over time.
In a related commentary, the study authors wrote, "This study makes a strong case for a potential effect of metformin on COVID-19.... and prompts a reevaluation of existing data in support of its use."
The Rainwater Charitable Foundation, The Parsemus Foundation, UnitedHealth Group, and Fast Grants funded the clinical trial. Drs. Bramante and Jacinda Nicklas' time was supported by the National Institutes of Health

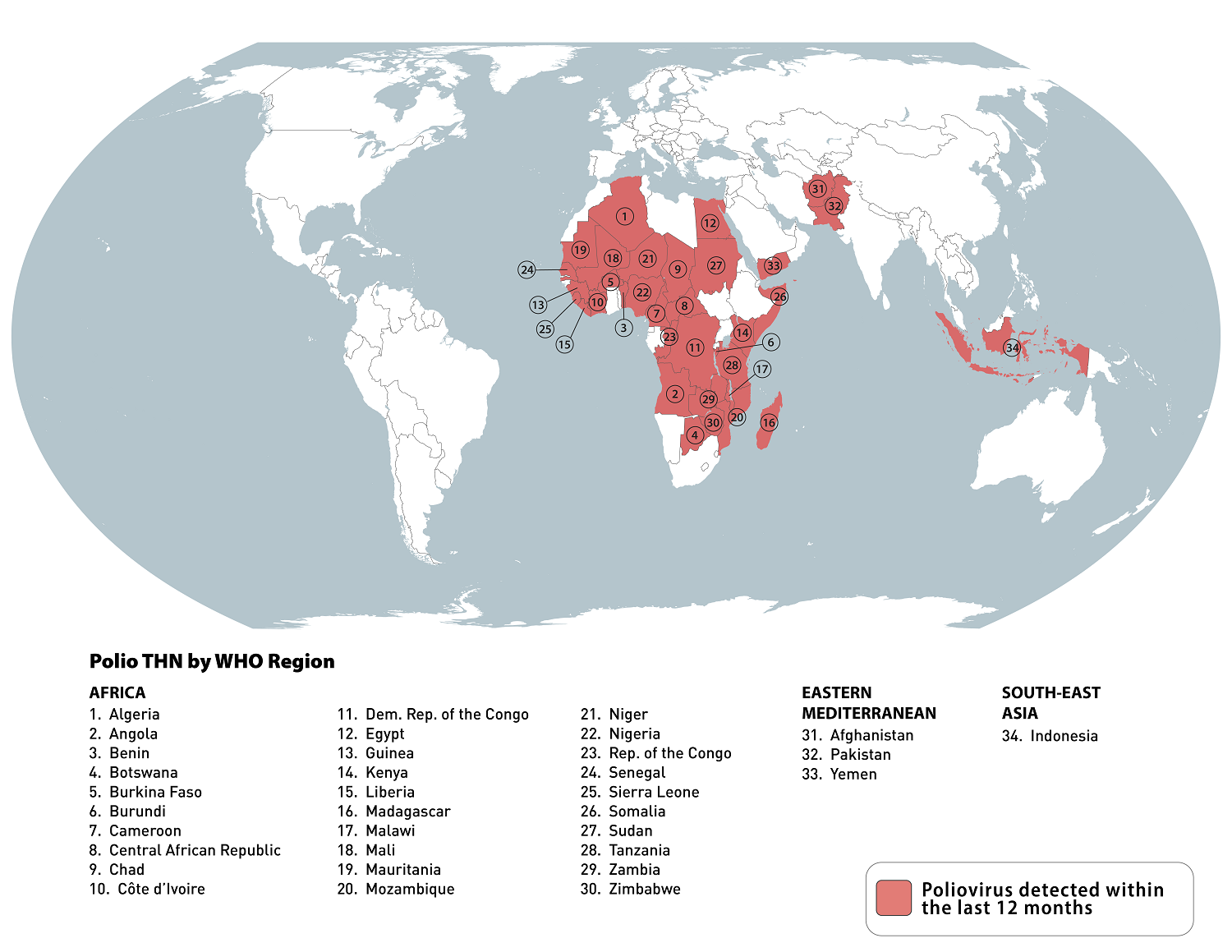
According to an updated Travel Health Advisory, there are now 34 destinations that may have circulating poliovirus.
As of May 3, 2024, the Global Polio Eradication Initiative confirmed various countries reported WPV1, cVDPV2, and cVDPV1 polio cases this year.
On April 26, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reissued its Level 2 - Practice Enhanced Precautions notice regarding recent polio outbreaks.
The CDC says that adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine before traveling to any destination listed. And to ensure that anyone who is unvaccinated or incompletely vaccinated completes the routine polio vaccine series before departing abroad.
In the U.S., polio vaccination services are generally available at health clinics and community pharmacies.
Previously, the World Health Organization (WHO) confirmed during the 38th meeting of the IHR Emergency Committee for Polio that the spread of the poliovirus remained a Public Health Emergency of International Concern and recommended its extension for a further three months to July 2024 to reduce the risk of the international spread of poliovirus.
Unfortunately, the U.S. is included in the WHO April 2024 notice.