Search API
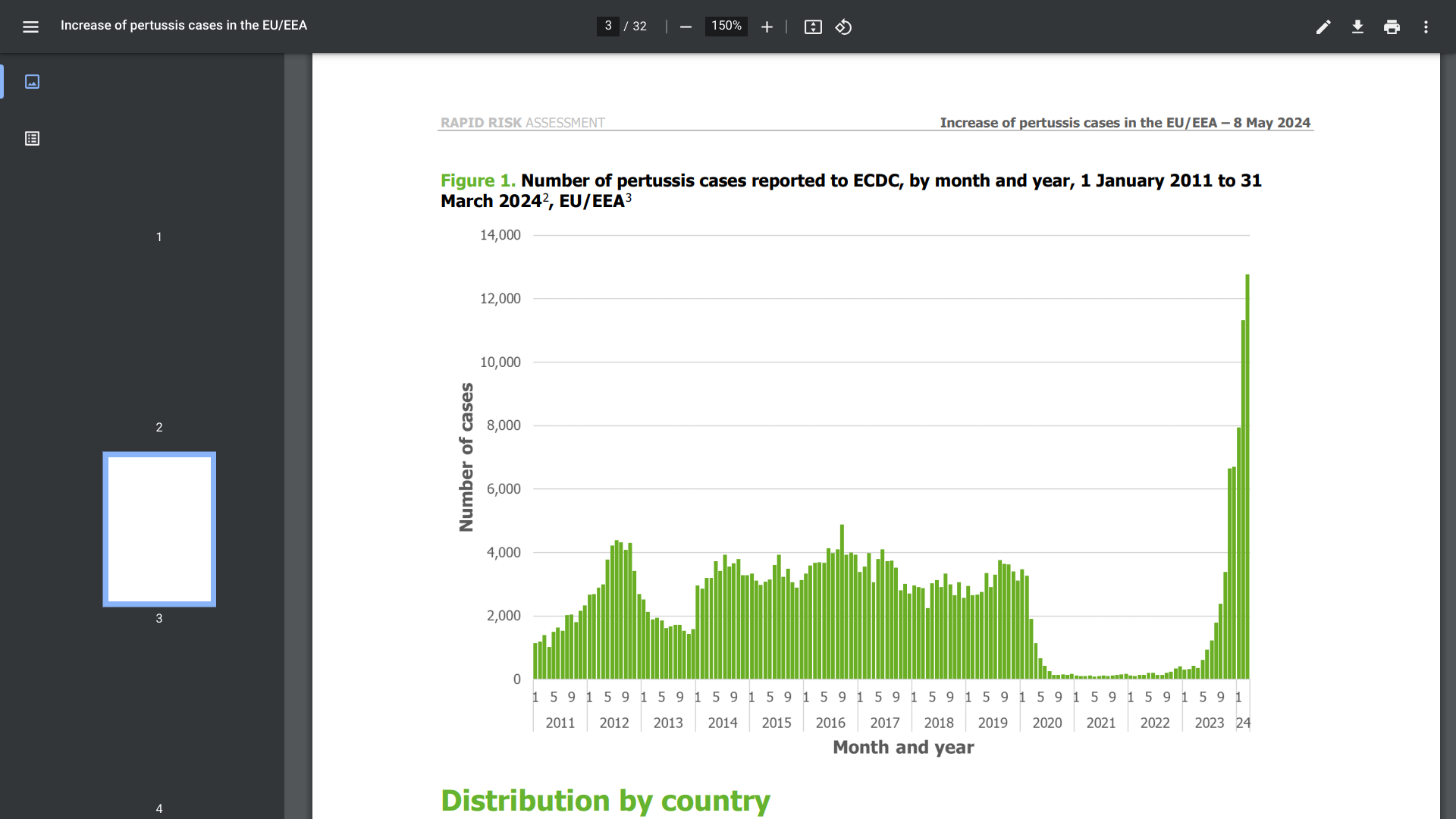
According to a new report from the European Centre for Disease Prevention and Control (ECDC), more than 25,000 cases of pertussis and 19 related deaths were reported in 2023, and more than 32,000 between January and March 2024.
The ECDC data announced on May 8, 2024, indicates a nearly 10-fold increase in pertussis (whooping cough) cases compared with 2022.
Historically, infants have had the highest incidence of pertussis reported in European countries. However, in 2023–24, an increase among infants has been observed, along with large increases in 10–14 and 15– 19-year-olds.
According to information from open sources, an increase in pertussis cases has also been reported in many countries in 2024, including Australia, Brazil, Canada, Israel, the United States, and the United Kingdom.
The primary objective of national pertussis immunization programs should be to curb morbidity and mortality in newborn infants.
To this end, and given the ongoing pertussis outbreaks, the ECDC encourages all public health authorities to focus on achieving and sustaining high vaccination coverage through timely and full completion of pertussis primary immunization series and subsequent boosters recommended nationally.
The World Health Organization and numerous agencies have approved various pertussis vaccines.
A recent study aimed to compare the positive benefits between two U.S. FDA-approved influenza antiviral medications.
Published in the journal Influenza and Other Respiratory Viruses on May 6, 2024, this study concluded that Xofluza (baloxavir marboxil, BMX) appears to be more effective than Tamiflu (oseltamivir, OTV) in lowering the secondary attack rate.
The Poisson regression modeling showed that in those cases treated with BXM and OTV, the benefits were 10.8% and 18.5%, respectively; the adjusted relative reduction was 41.8% (95% confidence interval: 1.0%–65.7%, p = 0.0456) greater with BXM than OTV.
These researchers wrote that one reason for this result may have been the difference in administration: a single dose of BXM versus twice daily for five days for OTV. Thus, BXM is expected to have higher compliance than OTV in children.
In summary, this post hoc analysis found that the secondary influenza illness attack rate was lower in household contacts exposed to BXM-treated than OTV-treated index cases.

According to Reuters reporting, AstraZeneca is voluntarily withdrawing its COVID-19 vaccine Vaxzevria as global demand has declined in 2024.
The University of Oxford, Serum Institute of India Pvt. Ltd., and AstraZeneca co-produced Vaxzevria COVID-19 (AZD1222). The European Medicines Agency initially authorized Vaxzevria in February 2021.
The London-based company indicated that the decision to pull Vaxzevria from the global market was due to the "surplus of available updated vaccines" adapted to newer and emerging variants of COVID-19, Reuters reported on May 6, 2024.
"We are incVaxzevria's role Vaxzevria played in ending the global pandemic. According to independent estimates, over 6.5 million lives were saved in the first year of use alone, and over three billion doses were supplied globally," AstraZeneca told The Telegraph in a statement.
Over the past month, other COVID-19 vaccine producers have reported significant decreases in consumer demand.
In the U.S., the Food and Drug Administration recently announced it intends to issue its recommendations for 2024-2025 COVID-19 vaccines in mid-June 2024.

ImmunityBio, Inc. announced today that the drug substance had been completed and successfully qualified for “fill finish” (filling vials and finishing packaging), sufficient for 170,000 doses of 400mcg ANKTIVA®.
ANKTIVA (nogapendekin alfa inbakicept-pmln) is the first U.S. FDA-approved immunotherapy for non-muscle invasive bladder cancer that activates natural killer cells, T cells and memory T cells for a long-duration response.
Coupled with the recent announcement of a partnership with the Serum Institute of India for enhanced BCG vaccine availability, this provides the Company with a significant initial supply of ANKTIVA for commercial and clinical trial use in advance of the full operation of the Company’s drug substance and fill-finish manufacturing plants in California and New York.
In 2024, Anktiva will be priced at $35,800 per dose. The cost of the BCG vaccine is additional.
Since the Company’s merger with NantKwest in 2021, ImmunityBio has made significant capital investments in personnel, plants, and equipment to ensure the global capacity of the ANKTIVA drug product for both the commercial launch and clinical trials in bladder cancer and other tumor types in its pipeline.
Both drug substance and drug product facilities are nearing completion to ensure sufficient capacity and multiple GMP manufacturing sites for ANKTIVA in its approved indication and for clinical trials and future indications.
“Our belief in the importance of this molecule and its potential to evolve immunotherapy to the next level guided our strategic plan to invest for the future with anticipation of ANKTIVA’s approval,” said Rich Adcock, CEO & President ImmunityBio, in a press release on May 7, 2024.
“I’m grateful for our employees and our investors who have supported and believed in our commitment to invest for our long-term vision and future.”
The Company is applying its science and platforms to treating cancers, including developing potential cancer vaccines, immunotherapies, and cell therapies that we believe will sharply reduce or eliminate the need for standard high-dose chemotherapy.
TCompany says these platforms and their associated product candidates are designed to be more effective, accessible, and easily administered than current standards of care in oncology and infectious diseases.

The U.S. Food and Drug Administration (FDA) today announced on X a change of date for its May 16, 2024, Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting.
The VRBPAC is now scheduled to meet on June 5, 2024, to recommend the inclusion of the SARS-CoV-2 coronavirus strain(s) in the 2024-2025 COVID-19 vaccines.
@FDACBER stated the VRBPAC will have more up-to-date information when discussing and making recommendations.
This advisory committee provides independent expert advice to the FDA on broad scientific topics or certain products to help the agency make sound decisions based on the available science.
As of 2024, about 50 COVID-19 vaccines have been approved globally since the pandemic began in late 2019. SKYCovione™ became the 12th, and CORBEVAX® the 13th COVID-19 vaccine granted authorization by the WHO.
Valneva SE today reported its financial results for the first quarter ending March 31, 2024, and provided corporate updates.
On May 7, 2024, the Company announced IXCHIQ®, the world's first and only licensed chikungunya vaccine, recorded initial sales of €0.2 million in the first quarter in the U.S.
In a press release, Peter Bühler, Valneva's Chief Financial Officer, commented, "The first quarter performance has been in line with our expectations. We are aiming to further capitalize on the travel industry recovery during the rest of the year (2024), including ramping up sales for IXCHIQ® to support our commercial sales growth while executing on our key R&D milestones."
IXCHIQ® is also under regulatory review in Canada, Brazil, and Europe, where it was granted accelerated assessment by the European Medicine Agency's Committee for Medicinal Products for Human Use. Decisions on these submissions are expected in 2024.
The U.S. Centers for Disease Control and Prevention (CDC) recently adopted the U.S. Advisory Committee on Immunization Practices recommendations, which include the chikungunya vaccine for persons aged ≥18 traveling to a country or territory with a chikungunya outbreak.
In addition, the CDC says the chikungunya vaccine may be considered for those traveling to a country or territory without an outbreak but with evidence of chikungunya virus transmission among humans within the last five years.
Persons aged >65 years, particularly those with underlying medical conditions, are likely to have at least moderate exposure* to mosquitoes OR Persons staying for a cumulative period of 6 months or more. Moderate exposure could include travelers with at least two weeks (cumulative) of exposure to mosquitoes in indoor or outdoor settings.
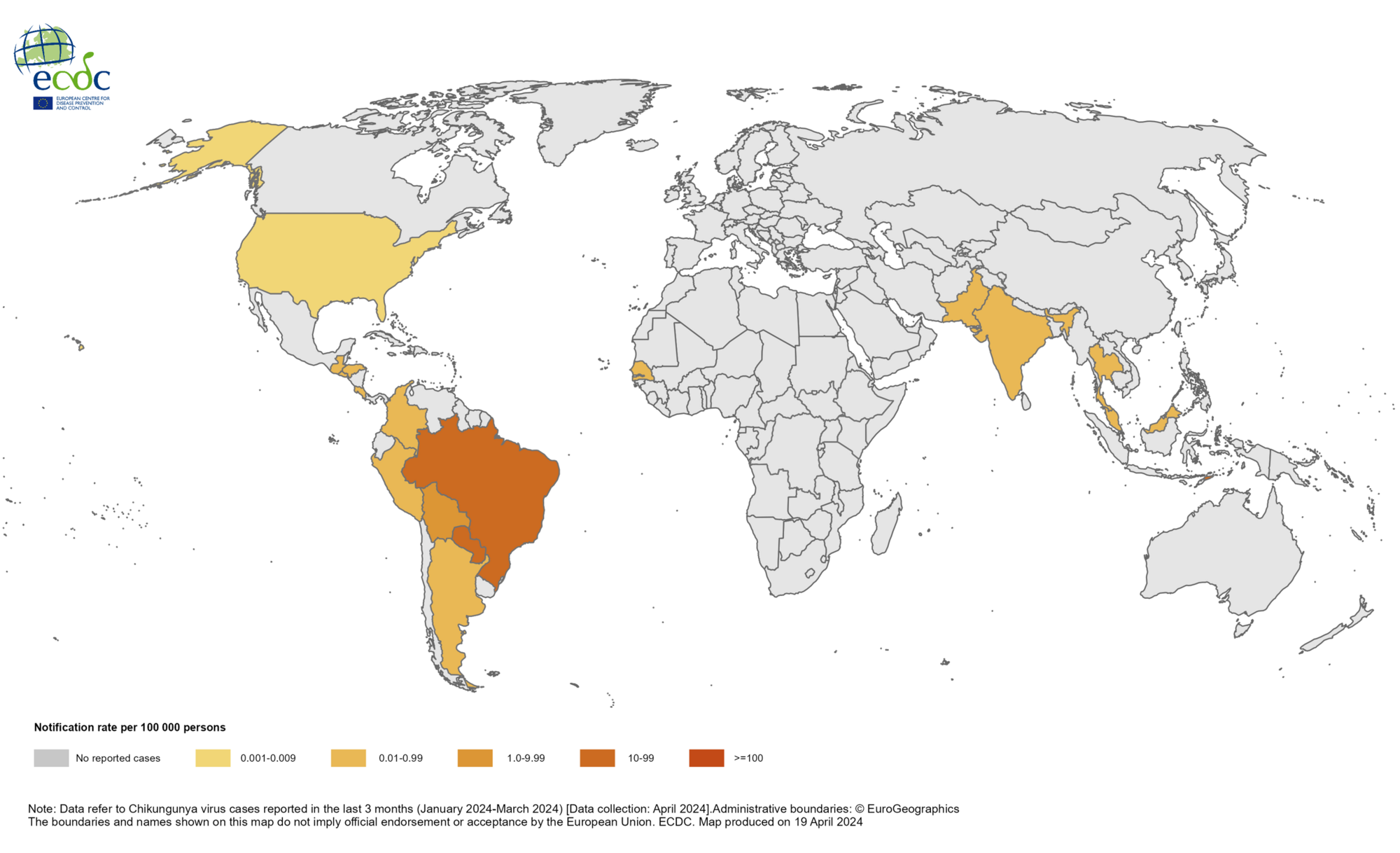
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus.
As of May 2024, the World Health Organization says chikungunya was identified in nearly 115 countries, primarily in the Region of the Americas.
According to the European Centre for Disease Prevention and Control, approximately 70,000 chikungunya cases and 15 deaths have been reported worldwide in 2024.
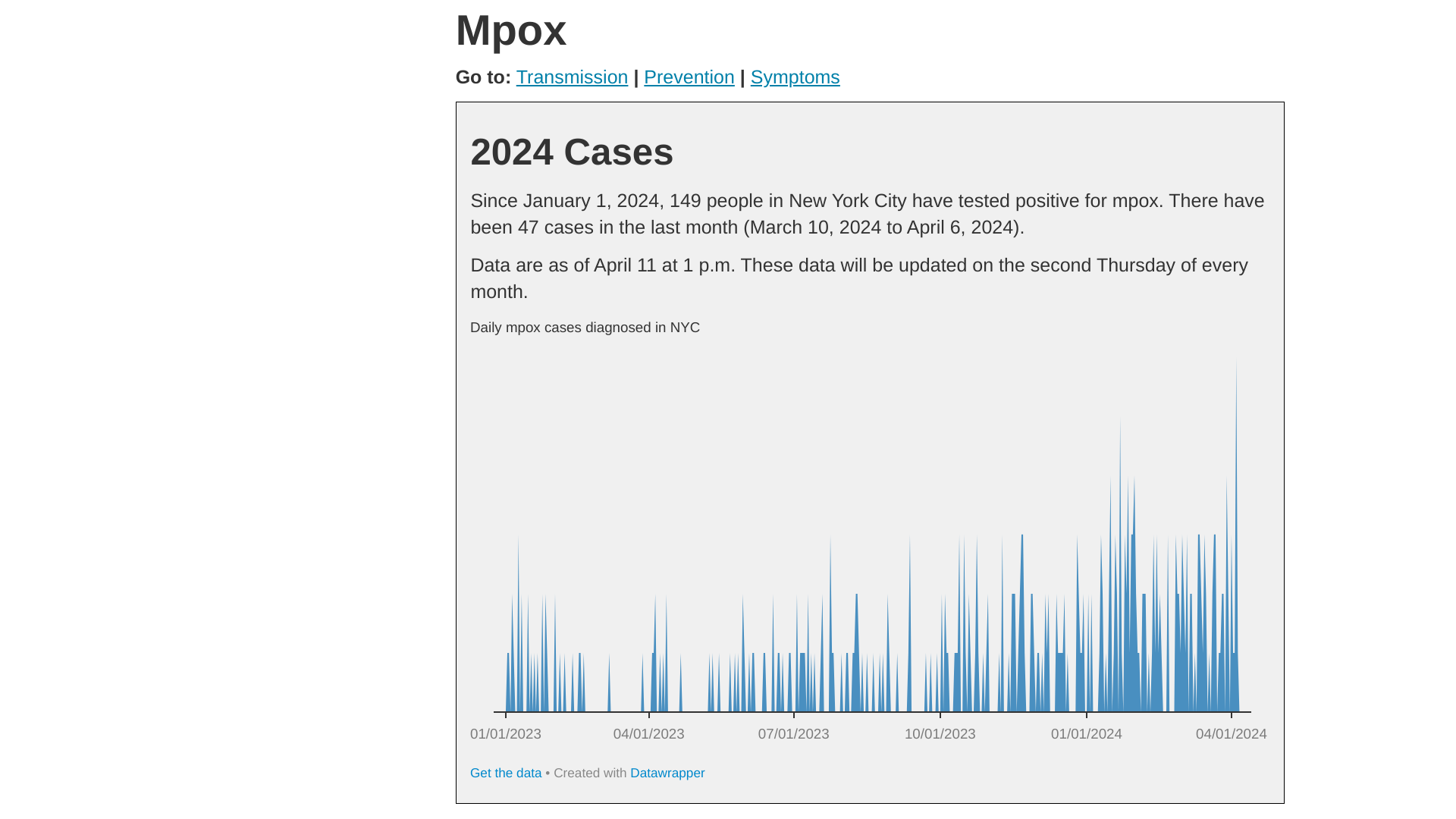
New York City's Deputy Commissioner, Division of Disease Control, Celia Quinn, MD, MPH, issued a statement confirming that mpox continues circulating in the city.
As of May 3, 2024, Health Advisory #12 reported the overall number of mpox cases is low compared to the 2022 outbreak. For most of 2023, the NYC averaged about 2 to 20 monthly cases.
Since October, there has been an average of 36 monthly cases, with a peak count of 51 in January 2024.
Of the 256 cases from October 2023 through April 15, 2024:
- 73% (188) were not vaccinated or had received only one dose,
- 94% were among men,
- 3.9% (10) of the infected people were hospitalized,
- Most were Black or Hispanic and between the ages of 25-44.
NYC confirmed the commercialization of the U.S. FDA-approved JYNNEOS® Mpox Smallpox vaccine is underway. As of May 2024, healthcare providers can order JYNNEOS through their distribution partners to make it available for at-risk individuals at local pharmacies.
Mpox is a contagious disease caused by the monkeypox virus. The U.S. Centers for Disease Control and Prevention and NYC continue to report only Clade II mpox cases in 2024.
Last week, the U.S. Government Accountability Office noted in a May 2, 2024 report that the Strategic National Stockpile (SNS) wasn't clear on how and from whom to request mpox supplies, including causing confusion and delays in mpox vaccine deliveries.
About 30% of U.S. jurisdictions during the mpox outbreak said the process for requesting SNS inventory did not follow written guidelines.