Search API
Due to the rise in dengue fever cases in the southern hemisphere this year and the start of the high transmission season in the northern hemisphere, the Pan American Health Organization (PAHO) is urging the Caribbean and Central American countries to enhance preventive measures against this mosquito-borne viral disease.
An Epidemiological Alert announced on May 28, 2024, reported that the Americas region has reported more than 8.1 million suspected dengue cases, a 3.3-fold increase compared to the same period in 2023.
More than 3,600 dengue-related deaths have been reported throughout the region.
Mexico has reported over 65,000 dengue cases, Guatemala over 12,000, Honduras over 20,000, and Panama over 5,800.
Meanwhile, countries and territories in the Caribbean have reported over 21,000 cases, representing a 5.7-fold increase compared to the corresponding period last year.
Previously, the U.S. CDC stated that most dengue cases reported in the 49 continental states occur in travelers infected elsewhere. As of May 2024, travel-related and locally acquired dengue outbreaks were reported in southeast Florida, New York, and Puerto Rico in 2024.
In today's Alert, the PAHO emphasizes the importance of timely clinical diagnosis, early identification of warning signs, and proper patient management to avoid severe cases and deaths.
In most cases, dengue has no symptoms and can present as flu-like. However, when symptoms do occur, they usually include high fever, headache, body aches, nausea and rash.
Although most people recover within one to two weeks, some can develop severe forms that require hospitalization. These can be fatal when not treated promptly and adequately.
Either of the two WHO-listed vaccines can also prevent dengue. Unfortunately, their availability will be limited in 2024.

The U.S. Centers for Disease Control and Prevention (CDC) today announced an outbreak of chikungunya in Malé and Hulhumalé regions of Maldives.
Crisis24 recently reported 389 total cases from March to May 11, 2024.
On May 28, 2024, the CDC Level 2 Travel Health Advisory recommended that adults traveling to a destination with a current chikungunya outbreak be vaccinated.
The U.S. FDA recently approved Valneva SE's IXCHIQ® monovalent, single-dose, live-attenuated chikungunya vaccine.
Furthermore, the CDC says if you are pregnant, reconsider travel to Maldives, particularly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
According to the CDC, newborns infected in this way or by a mosquito bite are at risk for severe illness, including poor long-term outcomes.
Additionally, people can protect themselves from chikungunya infection by preventing mosquito bites, including using insect repellent, wearing long-sleeved shirts and pants, staying in places with air conditioning, or using window and door screens.

CureVac N.V. today announced the dosing of the first participant in a Phase 2 study of the multivalent seasonal influenza vaccine candidate developed in collaboration with GSK.
The study will assess targeted optimizations for improved immune responses of the messenger ribonucleic acid (mRNA) vaccine candidate against the relevant influenza B strain.
The design of the multivalent vaccine candidate (FLU SV mRNA) has been changed to address all three World Health Organization (WHO)-recommended influenza strains and to exclude the influenza B/Yamagata lineage. The three remaining influenza strains include two influenza A strains and one influenza B strain.
This new Phase 2 study in the joint CureVac/GSK seasonal influenza program was initiated following interim data from the Phase 2 part of the ongoing combined Phase 1/2 study in seasonal influenza, which was reported on April 4, 2024.
Dr. Myriam Mendila, Chief Scientific Officer of CureVac, commented in a press release on May 28, 2024, "Historically, it's been challenging to target influenza B strains with a potent vaccine strategy. We are making progress in adapting and optimizing our clinical approach to address this challenge and improve performance against the remaining B strain."
The companies did not disclose a potential availability date for this new flu shot.
In response to the WHO's decision, various flu shot manufacturers intend to release modified vaccines in time for the 2024-2025 flu season in the United States. Last season, about 158 million flu shots were distributed, which was a decrease from the 2022-2023 flu season's 173 million.
Increased rates of sexually transmitted infections (STIs) are reported globally, and new interventions are needed, wrote researchers in a recent article published by The Lancet Infectious Diseases.
STIs continue to pose significant public health challenges, causing 2.5 million deaths each year, according to a new World Health Organization report.
On May 23, 2024, these France-based researchers wrote, 'We aimed to assess whether post-exposure prophylaxis (PEP) with doxycycline could reduce the incidence of chlamydia or syphilis (or both) and whether the meningococcal group B vaccine (4CMenB) could reduce the incidence of gonorrhea in this population.
Results from the ANRS 174 Doxyvac phase 3 clinical trial concluded Doxycycline PEP strongly reduced the incidence of chlamydia and syphilis.
These researchers said it did not show the efficacy of the 4CmenB vaccine for gonorrhea.
Still, doxycycline PEP should be assessed in other broader populations, and its effect on antimicrobial resistance carefully monitored.

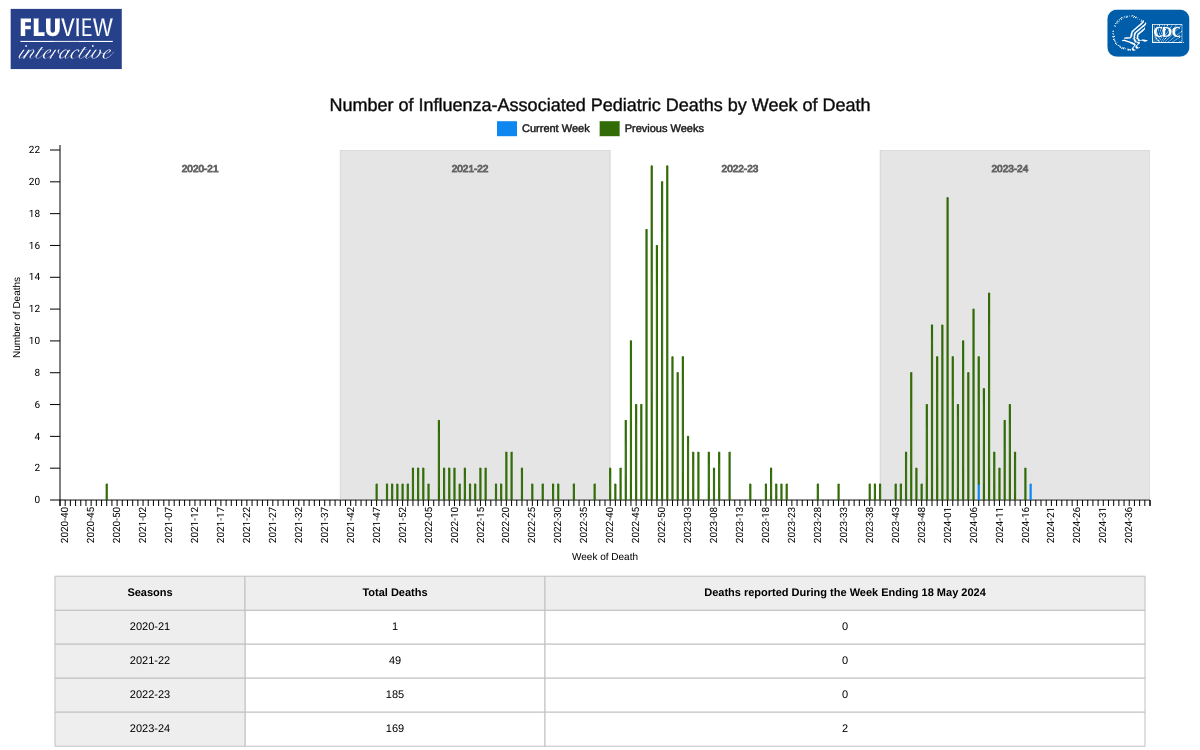
Nationally, outpatient respiratory illness remained stable and is below baseline for the seventh week in a row, according to Key Updates for Week #20 of the 2023-2024 flu season in the United States.
The U.S. Centers for Disease Control and Prevention (CDC) stated on May 24, 2024, that all ten regions are below their region-specific baselines.
Unfortunately, two influenza-associated pediatric deaths were reported last week, increasing this season's total to 169 pediatric deaths.
Last season, the CDC reported 185 pediatric deaths from influenza infections.
The CDC continues to encourage most children over six months of age to get an annual flu shot, either cell, egg, or nasal, which is offered at most clinics and pharmacies in the U.S.
A new framework was recently released by the World Health Organization (WHO) that will guide health authorities, communities, and other stakeholders in preventing and controlling mpox outbreaks, eliminating human-to-human transmission of the disease, and reducing spillover of the virus from animals to humans.
Mpox is a viral illness caused by the monkeypox virus (MPXV).
There are two different clades of the MPXV: clade I and clade II. Clade I outbreaks are deadlier than clade II outbreaks.
In the United States, clade II cases were reported to have a fatality rate of .002%.
A significant outbreak of the clade I virus in the Democratic Republic of the Congo (DRC) continues today, where cases have been detected for decades. Since the beginning of 2024, over 6,500 cases and 345 deaths have been reported in the DRC.
This data reflects a fatality rate of .05%.
As of May 26, 2024, most U.S. mpox cases continue to be in people who are not vaccinated or have only received one dose of the JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine. The U.S. CDC recommends that persons at risk for mpox exposure complete the 2-dose vaccination.
In the U.S., the JYNNEOS vaccine is offered at clinics and pharmacies in select cities.

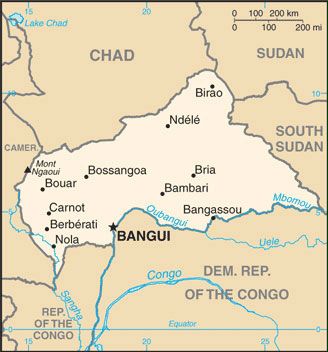
The World Health Organization (WHO) today announced that UNICEF successfully delivered over 43,000 doses of the R21/Matrix-M™ malaria vaccine to Bangui, Central African Republic.
As of May 24, 2024, 122,000 additional R21 doses are scheduled for delivery, funded by Gavi, the Vaccine Alliance.
The Central African Republic, with a population of over 5 million, is the first country to receive the R21 vaccine for routine childhood immunization. This marks another step forward in preventing the disease and saving children's lives.
The WHO says that along with the earlier WHO recommendation of the RTS,S vaccine, there is now sufficient vaccine supply to scale up malaria vaccination in Africa.
Chad, Cote d'Ivoire, Democratic Republic of Congo, Mozambique, Nigeria, South Sudan, and Uganda are preparing to receive R21 shipments.
Director of UNICEF Supply Division Leila Pakkala commented in a press release, "Previous concerns about supply meeting demand are firmly behind us. Our priority is for the vaccines to reach every child at risk."
The Central African Republic has one of the highest rates of malaria incidence globally. In 2022, an estimated 1,733,000 malaria cases were reported in the country, averaging about 4747 cases a day.
The disease also claimed around 5180 lives over the year, or 14 deaths each day.
Dr. Sania Nishtar, CEO of Gavi, the Vaccine Alliance, stated, "That is what matters most – that countries, where our vaccines can be most impactful, can access them, saving thousands of lives each year and offering relief to families, communities, and entire health systems."
On October 2, 2023, the WHO recommended R21 vaccination to prevent malaria in children. R21 is a protein-based vaccine developed by the University of Oxford, using Novavax AB's Matrix-M™ adjuvant technology.
"The R21/Matrix-M™ vaccine is a vital new tool to help stop the devastating health and economic impact of malaria on nearly half of the world's population, including the tragic loss of 1,300 children every single day," said John C. Jacobs, President and Chief Executive Officer, Novavax, on May 20, 2024.