Search API
The Centers for Disease Control and Prevention (CDC) says most dengue fever cases in the United States occur in areas where dengue is common, such as U.S. territories and a few states. Dengue virus is found in many tropical parts of the world, including the Caribbean, Central, and South America.
As of June 5, 2024, the CDC has reported over 100 additional dengue cases over the last month, which has increased this year's total to 1,706.
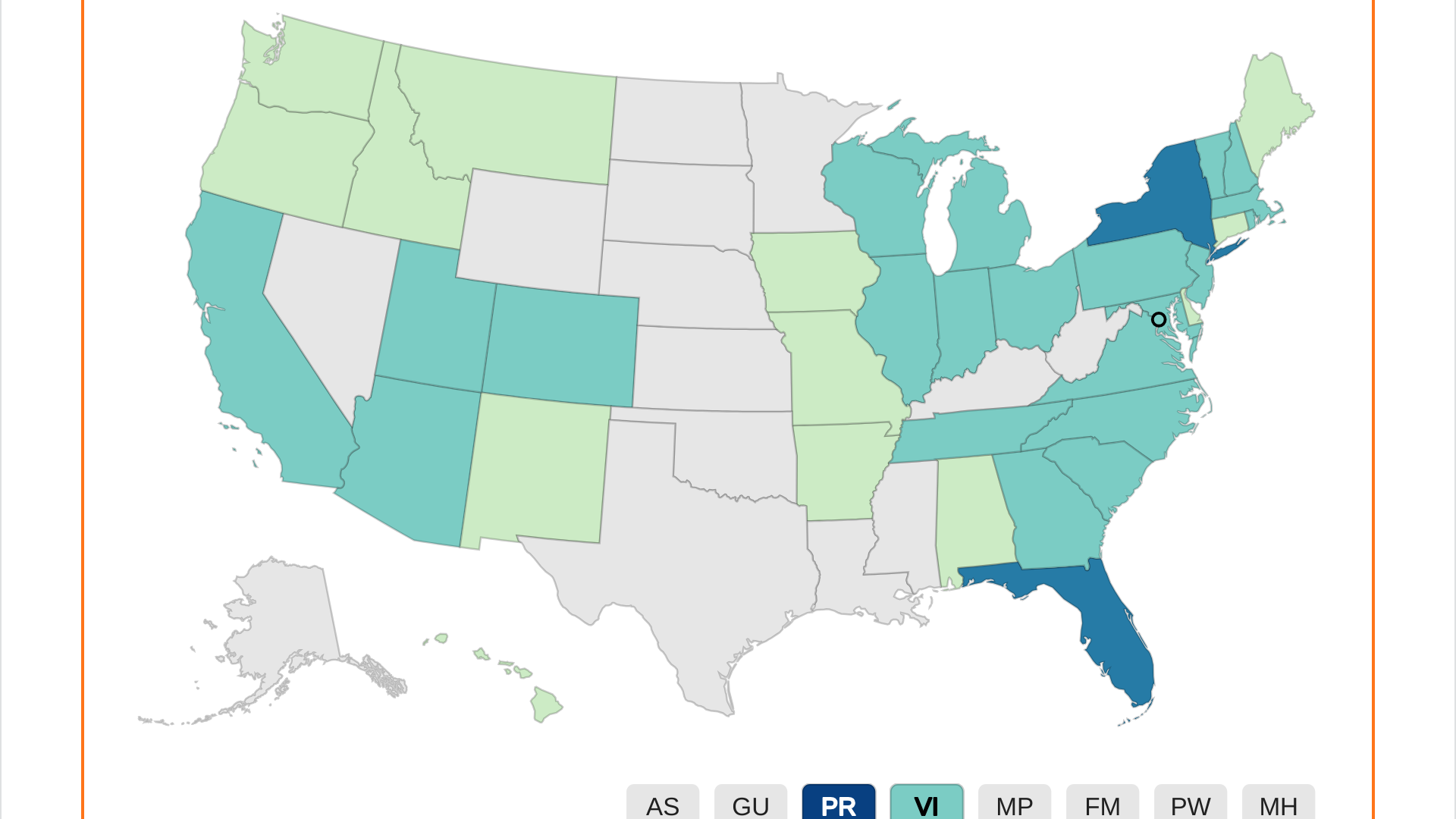
As of week #19 in 2024, Puerto Rico reported the most dengue cases, 1,107, with San Juan reporting 528 of these cases. In 2023, Puerto Rico reported 1,242 dengue cases.
Florida recently confirmed 198 travel-associated dengue cases in 2024, mainly by visitors from Brazil and Cuba. Seven cases of locally acquired dengue were reported from Miami-Dade and Pasco counties.
Additionally, the CDC reported New York's dengue outbreak has reached 119 cases in 2024.
Since the state of Texas has yet to post its dengue cases count for 2024, the CDC's overall total is expected to significantly increase. In 2023, Texas 67 travel-related dengue cases.
Dengue fever is a mosquito-borne, vaccine-preventable disease caused by the dengue virus. However, the WHO-approved dengue vaccines are not commercially offered in the U.S.
NeoImmuneTech, Inc. today announced that the European Medicines Agency (EMA) has granted Orphan Drug Designation (ODD) to NT-I7 for the treatment of Acute Radiation Syndrome (ARS).
This regulatory milestone follows the ODD granted to NT-I7 by the U.S. FDA for the same indication in November 2023.
ARS is a critical condition resulting from a high dose of radiation exposure, causing severe, sometimes fatal damage to the bone marrow and the immune system. No treatments are available that effectively promote T-cell recovery after such exposure.
NT-I7, a novel long-acting human interleukin-7 (IL-7), has shown promise to accelerate T cell reconstitution and enhance the immune response. It offers a potential solution for this unmet medical need. Clinical studies have shown that NT-I7 significantly boosts T cell counts while maintaining high safety and tolerability.
Luke Oh, Ph.D., President and Chief Executive Officer of NeoImmuneTech, said in a press release on June 4, 2024, "We are thrilled to see the deep interest of the scientific and regulatory community around the potential use of NT-I7 as a medical countermeasure in ARS."
"It is an important acknowledgment of the potential that NT-I7 holds in providing a beacon of hope for the treatment of ARS."
Examples of people who suffered from ARS are individuals exposed during the Hiroshima and Nagasaki atomic bombings and the Chernobyl Nuclear Power Plant incident firefighters.
NeoImmuneTech is actively developing NT-I7 in ARS through partnerships with the National Institute of Allergy and Infectious Diseases (NIAID) and Duke University. Recently, Duke University secured a $6 million grant from NIAID for NT-I7 development in ARS.

The Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet in an open session on June 5, 2024, to discuss and recommend selecting the 2024- 2025 Formula for COVID-19 vaccines.
VRBPAC has met multiple times since 2022 to discuss the selection of the Formula for COVID-19 vaccines. The U.S. FDA Briefing Document and Agenda for this new meeting were recently posted online.
The FDA has approved two COVID-19 vaccines for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 and older. In addition, the FDA currently authorizes three vaccines for use in the U.S. under emergency use authorization.
The U.S. COVID-19 emergency declaration was withdrawn on May 11, 2023.
Available data suggest that the current formula of COVID-19 vaccines should be updated for the anticipated 2024–2025 respiratory virus season in the U.S.
Based on the review of virus epidemiology data indicating the recent dominance of the JN.1 sublineage and data from post-monovalent XBB.1.5 human sera indicating a drop in neutralizing antibody responses against JN.1, the World Health Organization Technical Advisory Group on COVID-19 Vaccine Composition recommended that a monovalent JN.1 lineage component be included in the composition of COVID-19 vaccines (2024-2025 Formula).
This recommendation was made on April 26, 2024, before the dominance of the KP.2 sublineage in the U.S.
Initial neutralization data of various vaccine candidates are now available for both the JN.1 and KP.2 sublineages and will be presented along with current SARS-CoV-2 epidemiologic data for VRBPAC’s consideration.
To digitally attend this meeting, visit this YouTube link tomorrow: https://youtube.com/live/weaKQiFk_98

In a historic step towards mitigating the high incidence of malaria in South Sudan, the first consignment of the R21/Matrix-M™ vaccine malaria vaccine recently arrived in Juba.
The Ministry of Health received over 645,000 doses, which will be distributed to 28 counties.
South Sudan is grappling with one of the highest malaria incidence rates in the African region. In 2022, South Sudan had an estimated 2.8 million cases and 6,680 deaths.
“We are committed to reducing the impact of malaria and improving the health outcomes for our children,” said Honourable Yolanda Awel Deng, Minister of Health, in a UNICEF press release on May 31, 2024,
“The continued use of this vaccine, alongside other preventive measures such as insecticide-treated bed nets and timely access to medical care, will be instrumental in our efforts to eliminate malaria in South Sudan.”
With support from Gavi, UNICEF is working to procure enough malaria vaccines to immunize over 45 million children worldwide in the coming years.
As of June 3, 2024, malaria vaccines are not offered in the United States.

The U.S. Centers for Disease Control and Prevention (CDC) published a Morbidity and Mortality Weekly Report that confirmed Guillain-Barré syndrome (GBS) was identified as a potential safety concern following respiratory syncytial virus (RSV) vaccination.
On May 30, 2024, the CDC wrote that 'Findings are consistent with those from trials; reports of GBS (5.0 and 1.5 reports per million doses of Abrysvo and Arexvy vaccine administered, respectively) were more common than expected background rates.
Two deaths among RSV vaccine recipients who had been diagnosed with GBS were reported.
The CDC, the U.S. FDA, and the Centers for Medicare & Medicaid Services are conducting population-based surveillance assessments of RSV vaccine safety and risks for GBS. Findings from these studies will help guide the development of Advisory Committee on Immunization Practices recommendations.
About 10 million older adults have been vaccinated against RSV since August 2023.
As of June 3, 2024, three approved RSV vaccines for adults and one monoclonal antibody for infants are available in the U.S.

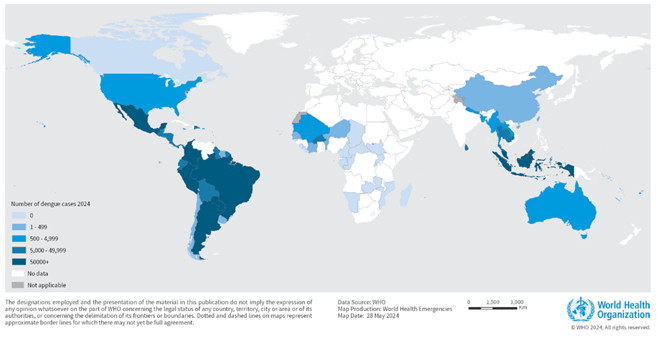
The World Health Organization (WHO) recently published a Disease Outbreak News highlighting the global dengue fever outbreak in 2024.
On May 30, 2024, the WHO reported that 90 countries have known active dengue transmission. The WHO confirmed that over 7.6 million dengue cases, including over 16,000 severe cases and over 3,000 deaths, were reported in 2024.
While a substantial increase in dengue outbreaks has been reported globally in the last five years, this increase has been particularly pronounced in the Region of the Americas. By the end of April 2024, the number of cases had already exceeded seven million, surpassing the annual high of 4.6 million cases in 2023.
In the U.S., the Centers for Disease Control and Prevention recently reported 41 jurisdictions 1,611 dengue cases in 2024.
To help prevent the impact of this mosquito-transmitted disease, the WHO has Listed two dengue vaccines, which are available in certain specific requirements in various countries as of June 2, 2024.
Jeri Beales, MSN, RN with Destination Health Clinic in Massachusetts, reminds travelers, "Unfortunately, there is no dengue fever vaccine approved for U.S travelers, so the only prevention method is to avoid mosquito bites."
Beales informed Vax-Before-Travel travelers should "Pack a quality insect repellent and apply to your skin before going outdoors. A DEET product with 20-50% concentration works great for keeping mosquitoes away."
"Remember to put sunscreen on first, then the DEET repellant on second to skin not covered by clothing. To add an extra layer of protection, spray your clothing with another insect repellant called permethrin."
"By pre-treating clothing before your departure, you don't have to pack a large spray bottle, and most permethrin brands protect for up to 6 weeks even with washing."
According to the Global Polio Eradication Initiative (GPEI), five counties reported new cases involving vaccine-derived poliovirus types.
The GPEI's latest weekly update confirmed Angola reported its second circulating vaccine-derived poliovirus type 2 (cVDPV2) case of 2024, and Chad, the Democratic Republic of the Congo (DRC), Niger, and Nigeria also reported cVDPV2 cases as of May 29, 2024
Additionally, the DRC reported two more cases of circulating vaccine-derived poliovirus type 1.
The World Health Organization (WHO) confirmed in April 2024 that the spread of the poliovirus remained a Public Health Emergency of International Concern and recommended its extension through July 2024 to reduce the risk of the international spread of poliovirus outbreaks.
In May 2024, the U.S. Centers for Disease Control and Prevention (CDC) issued an updated Global Polio Alert - Level 2, Practice Enhanced Precautions Travel Health Notice regarding polio outbreaks and poliovirus detections in 34 countries.
The African country Angola recently reported two cases of Acute Flaccid Paralysis (AFP). These AFP cases are the first in Angola since the 2019-2020 polio outbreak, which recorded 124 cases.
Furthermore, 36 potential AFP cases are awaiting classification, which may or may not be related to the polio virus.
As of June 2024, both the WHO and CDC recommend polio vaccinations and booster doses for under-immunized people visiting outbreak areas.
In the U.S., IPV vaccines have been offered since 2000, and over 1 billion nOPV2 vaccine doses have been offered in African countries.

The Chicago Department of Public Health (CDPH) today declared the recent outbreak of measles in the City of Chicago officially over. Two full incubation periods (42 days) have passed without any new measles cases.
On March 7, 2024, CDPH confirmed the first case of measles in Chicago in five years. In total, 64 individuals tested positive for measles, 57 of whom were associated with a shelter setting.
Over 30,000 measles-mumps-rubella vaccine doses have been administered to Chicago residents.
CDPH Commissioner Dr. Olusimbo Ige said in a press release on May 31, 2024, “Our goal is the elimination of measles in Chicago and everywhere, and with proper vaccination coverage, we know that zero is possible.”
For those who are traveling internationally, additional measles vaccinations may be recommended. Talk with your healthcare provider (doctor, nurse, pharmacist) before traveling about vaccinations you and your family may need, wrote CDPH.
As of May 30, 2024, the U.S. CDC reported a total of 146 measles cases in 21 jurisdictions: Arizona, California, Florida, Georgia, Illinois, Indiana, Louisiana, Maryland, Michigan, Minnesota, Missouri, New Jersey, New York City, New York State, Ohio, Pennsylvania, Vermont, Virginia, Washington, West Virginia, and Wisconsin.
There have been 11 outbreaks (three+ cases) reported in 2024.
Globally, measles cases have been confirmed in 52 countries in the past year by the CDC.