Search API
A position paper published online in the Annals of Allergy, Asthma & Immunology reviewed the safety of administering live vaccines to people currently treated with Dupilumab®, a biologic therapy.
Dupilumab is a monoclonal antibody that targets the interleukin (IL)-4 receptor alpha subunit, thus blocking the effects of IL-4 and IL-13. It has shown efficacy in treating various conditions, including asthma, atopic dermatitis, eosinophilic esophagitis, and others.
Because Dupilumab is now approved for use in patients from six months of age for the treatment of atopic dermatitis, a reported contraindication is now posing a clinical dilemma for patients and clinicians.
After a systematic review of available data, this panel concluded on June 5, 2024, that stopping treatment with Dupilumab is unnecessary to administer live vaccines.
However, the panel recommends that doctors and patients/parents engage in shared decision-making to determine the timing and necessity of administering live vaccines to individuals being treated with Dupilumab or whether treatment should be paused.
As a clinical resource, board-certified allergists/immunologists have special training in managing and treating people with asthma or allergies who may benefit from this medication.
According to MedlinePlus, there are five types of vaccines are currently available, including live virus vaccines. These vaccines use the weakened (attenuated) form of the targeted virus. The MMR and the varicella (chickenpox) vaccines are examples.

Vaxxinity, Inc. announced today that the journal Nature Medicine has published groundbreaking exploratory data from the Company's Phase 1 clinical trial of UB-312 in patients with Parkinson's disease (PD).
The Phase 1 successfully met its primary outcome measures, demonstrating that UB-312 was generally well-tolerated and induced anti-aggregated α-synuclein (αSyn) antibody responses in healthy volunteers and PD patients. Specifically, 12 of 13 PD patients who completed dosing developed anti-αSyn antibodies.
And UB-312-induced antibodies significantly decreased levels of αSyn, a key pathology in PD and other synucleinopathies. This suggests that UB-312 can help to eliminate the buildup of harmful, toxic forms of the protein αSyn in the brain.
Furthermore, patients with detectable UB-312-induced antibodies in cerebrospinal fluid exhibited significant improvement in motor experiences of daily living as measured by the MDS-UPDRS Part II, a commonly accepted clinical scale.
This announcement marks a potentially significant milestone in pursuing innovative PD care.
"UB-312 has the potential to become an important and potent disease-modifying therapy for Parkinson's disease. It would be truly amazing if we could vaccinate people against Parkinson's disease in the future!" said Professor Geert Jan Groeneveld, neurologist and principal investigator of the Phase 1 clinical trial performed at the Centre for Human Drug Research in Leiden, the Netherlands, in a press release on June 20, 2024.
Parkinson's disease, a progressive neurodegenerative condition, currently lacks an approved disease-modifying treatment. Alpha-synuclein, a key protein in PD pathology, forms aggregates known as Lewy bodies that contribute to neuronal degeneration.
This active immunotherapy medicines research was funded by the Michael J. Fox Foundation, assessing target engagement in collaboration with the Mayo Clinic and UTHealth Houston in Texas.

Recce Pharmaceuticals Ltd. today announced that its primary anti-infective candidate, RECCE® 327 (R327), was added to the World Health Organization's (WHO) report on Antibacterial Agents in Clinical Development and Preclinical Development.
The updated WHO report covers traditional and non-traditional antibacterial agents in development worldwide and evaluates to what extent the present pipeline addresses infections caused by priority pathogens.
R327 has been defined by the WHO as an ATP production disruptor and is the only compound under this category.
When targeted as the main mechanism of action, not secondary to other cell perturbation mechanisms, disruption of ATP production in bacterial cells has the potential to confer activity against both Gram-positive and Gram-negative pathogens.
Recce Pharmaceuticals CEO James Graham commented in a June 18, 2024, press release, "We are pleased that R327 has been included in the list of antibacterial products aimed at tackling the urgent global health threat posed by antibiotic resistance."
"There is a demand for new antibiotic therapies, and this report further showcases R327's potential as a novel treatment for a broad range of life-threatening and resistant bacteria."
Recce's anti-infective pipeline aims to address synergistic, unmet medical needs, such as urinary tract infections (UTIs).
UTIs are among the most common infectious diseases in the pediatric, female, and male populations.
The company anticipates releasing data in 2024 that is expected to pave the way for a Phase II UTI/Urosepsis efficacy trial, potentially establishing R327 as a frontline UTI treatment.

Clover Biopharmaceuticals, Ltd. today announced positive preliminary immunogenicity and safety data in the older adult cohort from its Phase 1 clinical trial evaluating SCB-1019, the company's bivalent RSV prefusion-stabilized F (PreF)-Trimer subunit vaccine candidate, which is based on Clover's Trimer-Tag vaccine technology platform.
Results indicate that SCB-1019 could potentially have a differentiated and favorable safety and reactogenicity profile compared to currently approved oil-in-water adjuvanted4 and/or mRNA5-based RSV vaccines.
"As the first RSV PreF vaccine candidate developed in China to enter the clinical trial stage and generate clinical data, SCB-1019 .... demonstrate broad and significant neutralizing antibody responses against both RSV-A and RSV-B as well as a favorable tolerability profile," said Joshua Liang, Chief Executive Officer & Board Director of Clover, in a press release on June 18, 2024.
"We look forward to the full clinical readout by the end of 2024 to support further development and strengthen our potentially differentiated profile for markets globally."
Preliminary results for RSV-neutralizing antibodies (nAbs) and safety for SCB-1019 at the selected dose level are summarized below:
Immunogenicity Results:
RSV-A nAbs: SCB-1019 induced geometric mean titers (GMTs) in RSV-A nAbs of up to 7,906 IU/mL compared to 1,078 IU/mL for placebo at Day 28.
RSV-B nAbs: SCB-1019 induced GMTs in RSV-B nAbs of up to 46,674 IU/mL compared to 12,185 IU/mL for placebo at Day 28.
Geometric Mean Fold Rise (GMFR): High baseline nAb titers at Day 0 (pre-vaccination), especially to RSV-B, were observed, potentially reflecting recent outbreaks near the clinical trial sites.
Thus, sub-analysis in subjects with the lowest quartile baseline nAb titers was performed: GMFRs for SCB-1019 were up to 8-fold for RSV-A nAbs and 11-fold for RSV-B nAbs at Day 28 compared to Day 0 (pre-vaccination). No increases in RSV-A or RSV-B nAbs were observed for the placebo on Day 28.
nAb results across both RSV-A and RSV-B appear to be in line or potentially favorable compared to other protein subunit RSV PreF vaccines.
Given that other monovalent RSV-A vaccines have previously observed lower immune responses and/or efficacy against RSV-B, we continue to support Clover's bivalent RSV-A/B approach, wrote the company.
Results further confirm that Clover's PreF antigens in SCB-1019 are in the stabilized prefusion and trimeric fo. This is additionally supported by exploratory immunogenicity results demonstrating significant increases in Site Ø and Site V nAb-competitive titers.
Safety & Reactogenicity Results: SCB-1019 was generally well-tolerated. Local and systemic adverse events (AEs) were generally mild and comparable to saline placebo. No serious adverse events, adverse events of special interest, or AEs leading to discontinuation were observed.
These preliminary results in older adults & elderly cohort (aged 60-85) are consistent with the positive results in younger adults (aged 18-59) announced earlier this year.
As of June 18, 2024, the U.S. FDA has approved three RSV vaccines and one monoclonal antibody (Beyfortus) for infants for the 2024-2025 RSV season.

Bavarian Nordic A/S today announced the completion of the rolling submission process with the U.S. Food and Drug Administration (FDA) for a Biologics License Application (BLA) for the licensure of its CHIKV VLP vaccine candidate for immunization against chikungunya virus infection in individuals 12 years of age and older.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization against chikungunya disease.
Initiated in April 2024, with acceptance from the FDA, the BLA could support a potential vaccine approval in the first half of 2025.
Bavarian Nordic also intends to submit a Marketing Authorisation Application (MAA) with the European Medicines Agency (EMA) by the end of the first half of 2024. The MAA has already been granted accelerated assessment, which means the CHIKV VLP vaccine could obtain approval from the European Commission in the first half of 2025.
“The completion of the BLA submission marks a significant milestone in the development of our CHIKV VLP vaccine and represents an important contribution to the development of preventative solutions for individuals 12 years of age and older at risk of chikungunya virus from bites by infected mosquitos. With the near-term anticipated MAA submission to EMA, we are looking towards potential approval of the vaccine in the first half of 2025 and subsequent launch in both the U.S. and E.U.,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on June 17, 2024.
In the United States, the FDA has approved one chikungunya vaccine.
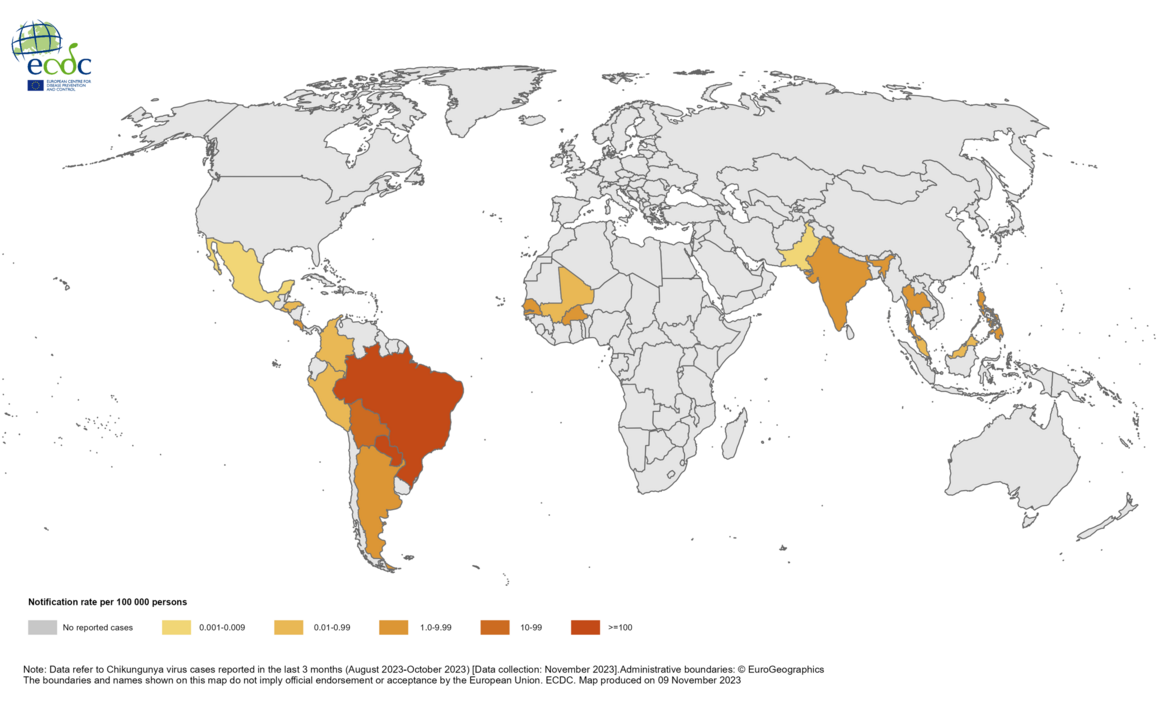
Chikungunya is a mosquito-borne viral disease that causes fever and severe joint pain. The World Health Organization (WHO) says the disease was first recognized in 1952. It is a ribonucleic acid virus belonging to the alphavirus genus of the family Togaviridae.
The WHO says chikungunya outbreaks have been identified in nearly 115 countries, primarily in the Region of the Americas. Most chikungunya cases in the contentital USA are travel related.
Today's dynamic digital vaccine news typically involves fact-checking by trusted third parties, but it rarely includes a manufacturer openly responding to a potentially biased article.
Recently, the news industry has been challenged in its ability to comprehend vaccine-related clinical trials and technical journal articles.
For example, on June 16, 2024, The Economic Times published a headline stating, 'Bharat Bio's Rotavirus vaccine Rotavac may be unsafe for children: Study.'
Later that day, the company posted on X its response....Unfair Journalistic Practices and Breach of Ethics by The Economic Times.
While this vaccine efficacy debate may be illuminated on social media for days and probably never settled, vaccine hesitancy and reduced trust in the healthcare delivery system will continue.
In most countries, government agencies, such as the U.S. FDA, play vital roles in communicating virology (vaccines, immunotherapies, gene therapy, monoclonal antibodies) risks and benefits, not media-centric fiction.

Novavax, Inc. today announced that it has submitted an amendment to its Emergency Use Authorization to the U.S. Food and Drug Administration (FDA) for its updated JN.1 COVID-19 vaccine (NVX-CoV2705) for individuals aged 12 and older.
The submission aligns with guidance from the U.S. FDA, the European Medicines Agency, and the World Health Organization to target the JN.1 lineage during late 20204.
As discussed at the recent FDA meeting, targeting JN.1, the parent strain of the most common currently circulating SARS-CoV-2 virus variants has a public health benefit.
Novavax's JN.1 vaccine has demonstrated broad cross-neutralizing antibodies against multiple variant strains, including KP.2 and KP.3, indicating the potential to protect against forward drift variants.
"Novavax is committed to having a protein-based COVID-19 option available at the start of the vaccination season, which is critical because research suggests that providing vaccine choice, along with healthcare provider recommendations, may help improve vaccination rates," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on June 14, 2024.
Nonclinical data have demonstrated that Novavax's JN.1 vaccine induces broad neutralization responses to JN.1 lineage viruses, including those containing the F456L and R346T mutations, and to "FLiRT" and "FLuQE" variants.
Novavax's vaccine also produces conserved polyfunctional, Th1-biased CD4+ T cell responses to a range of JN.1 lineage variants.2 Novavax's updated JN.1 COVID-19 vaccine targets the "parent strain" of KP.2 and KP.3.
Novavax intends to have doses in the U.S. for distribution by mid-July. Upon FDA authorization and U.S. CDC recommendation, Novavax is preparing to deliver to U.S. customers promptly. Novavax is also working with other regulatory authorities globally to authorize or approve its JN.1 COVID-19 vaccine.