Search API
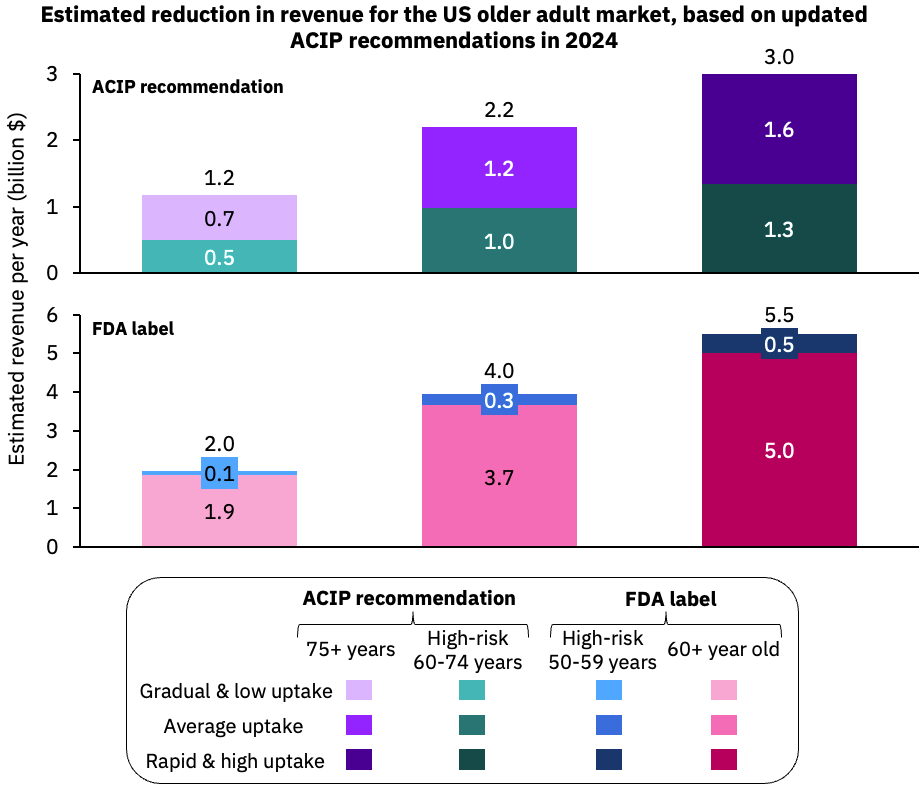
The market research firm Airfinity Limited recently lowered its sales projections for Respiratory Syncytial Virus (RSV) vaccines for older adults in the United States from $4.7 billion annually to $1.7 billion by 2030.
Airfinity’s estimate framework announced on July 23, 2024, expects 2024 revenues to reach $2.2 billion.
Which is reduced from 2023, when RSV vaccine revenues totaled about $ 2.4 billion.
This change in forecast follows new recommendations from the U.S. CDC vaccine advisory committee that RSV vaccines be offered only as a single lifetime dose for older adults. This reduces the estimated eligible population to about 46 million people.
Furthermore, the CDC has not announced a decision on potential booster doses.
However, future dosing recommendations could change as long-term efficacy and safety data emerge. Airfinity wrote that applying scenario frameworks for a booster every two or three years could increase the U.S. market's value to $6.6 billion or $5.2 billion, respectively.
Airfinity’s RSV Lead Isabella Huettner commented, “U.S. market share estimates are difficult to anticipate at this point with different scenarios being possible. Based on current data, GSK (AREXVY™) appears to be most likely to capture the majority of the market in the long term due to promising efficacy and durability."
As of July 28, 2024, three RSV vaccines are approved for use in the U.S.
Previously, Beyfortus™, the first approved extended half-life monoclonal antibody offering passive immunization to prevent lower respiratory tract infections in infants caused by RSV, is expecting a 'blockbuster' performance in the second half of 2024.
Beyfortus produced revenues of €547 million in 2023.
GlobalData plc previously issued a sales forecast indicating Beyfortus could reach global sales of $1.27 billion in 2029.
The U.S. Department of Health and Human Services recently published an amendment to a 2013 emergency declaration under the Food, Drug, and Cosmetic Act that broadens the scope of the agency's assistance in facilitating certain medical countermeasures in response to a public health emergency, such as a pandemic.
The new declaration enables the U.S. Food and Drug Administration (FDA) to extend the expiration date of certain medical products and allow HHS to issue an emergency use authorization for unapproved drugs, devices, or products, among other actions, including vaccines.
As of July 18, 2024, the amendment now applies to pandemic influenza A viruses and others with pandemic potential, such as the current H5N1 strain of avian influenza (bird flu, cow flu).
Previously, the declaration specifically covered just the H7N9 strain.
In June 2024, the U.S. Administration for Strategic Preparedness and Response announced the Pandemic Influenza Preparedness and Response Strategy. The Strategy outlines how the U.S. government will leverage existing infrastructures and capabilities to respond to the current Highly Pathogenic Avian Influenza H5N1.
In April 2024, the FDA's Dr. Peter Marks informed the media that the U.S. stockpile of avian influenza-specific vaccines would work well if deployed. Over the past few years, the U.S. has invested hundreds of millions of dollars in various bird flu vaccines.
As of July 27, 2024, FDA-approved avian influenza vaccines are not commercially available in the U.S.

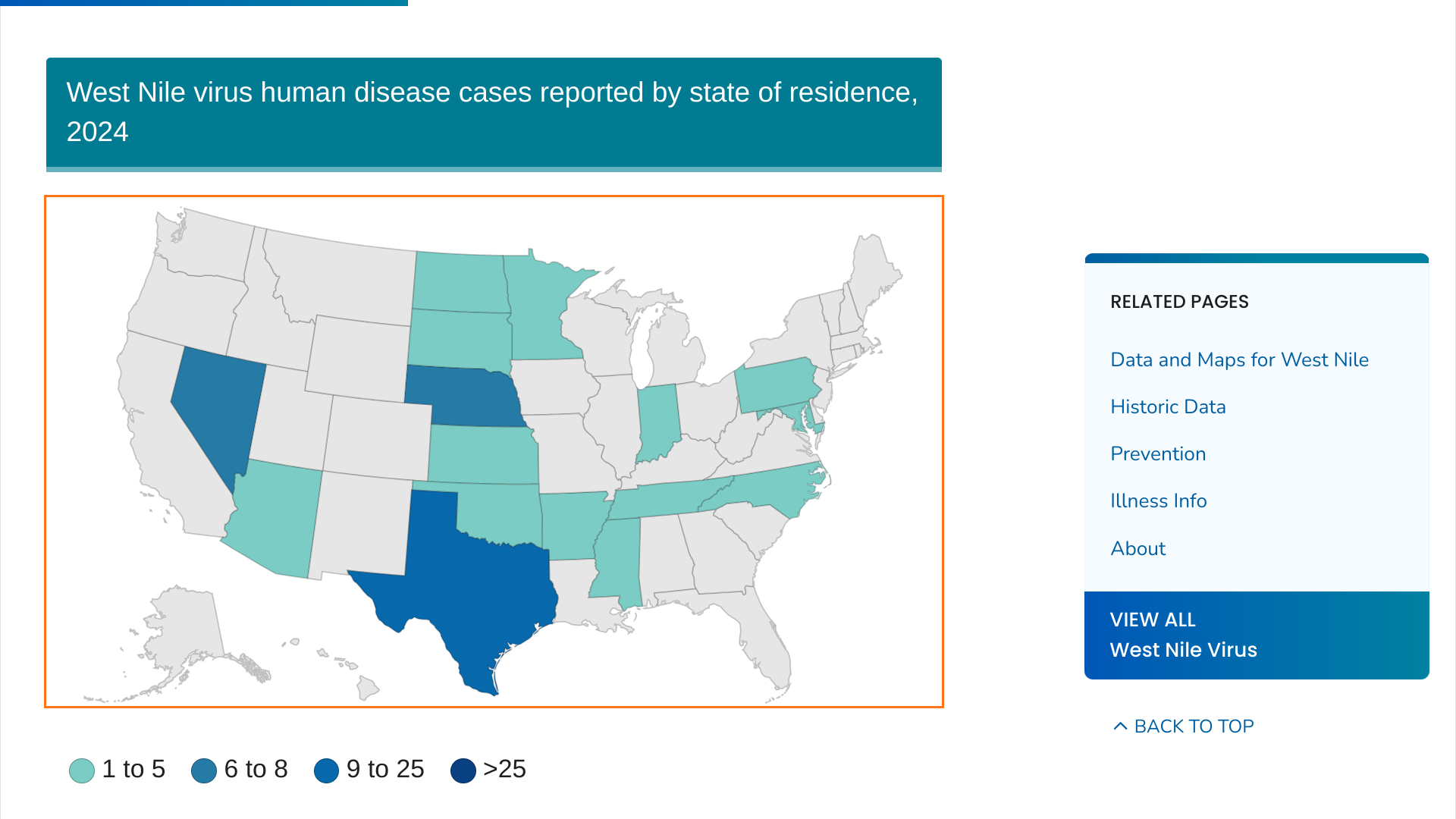
Harris County Public Health’s (HCPH) Mosquito and Vector Control Division today reported a significant increase in West Nile virus (WNV), which is the leading cause of mosquito-borne disease in the continental United States.
On July 26, 2024, seven human cases of WNV were reported to HCPH in unincorporated Harris County (outside the City of Houston).
Additionally, 520 positive mosquito samples were identified across 168 of its 268 operational areas in Harris County, which has a population of about 4.9 million.
HCPH urges residents to protect themselves and their loved ones against this mosquito-transmitted illness.
As of July 23, 2024, the U.S. CDC reported 45 WBV disease cases and 24 WNV neuroinvasive disease cases from 19 states this year.
Furthermore, the CDC says there are no vaccines or medicines to prevent WNV disease.
Several vaccine candidates, including live attenuated chimeric, DNA (first and second generation), recombinant subunit, and inactivated whole-virus vaccines have been the subject of human clinical studies.
The Minnesota Department of Health (MDH) recently confirmed three additional measles cases in unvaccinated children in Anoka, Hennepin, and Ramsey counties.
MDH stated that based on current information, these cases are not directly linked and have not traveled, so there is concern for the possible spread of measles in the community.
As of July 26, 2024, Minnesota has confirmed 15 measles cases in 2024, and is an increase compared to other years. All the cases have occurred among unvaccinated children.
Minnesota isn’t the only state to have seen an increase in measles cases. Just to the south, Chicago, Illinois, reported a significant outbreak (64 cases) this year.
MDH is working with local health departments and other locations to notify people who may have been exposed directly. However, health officials note that anyone not vaccinated against measles could be at risk and should watch for symptoms of measles.
“Measles spreads easily, and it finds those who are vulnerable,” said Jessica Hancock-Allen, infectious disease division director at MDH, in a press release.
“That is why families need to ensure their children are up to date on their immunizations to protect them from this potentially serious disease.”
"The best way to prevent measles is through immunization."
Measles vaccines are generally available at clinics and pharmacies throughout the U.S.

As flu shots arrive in local pharmacies next month, Canadians will have different vaccines to choose which is best for their needs.
To assist this decision process, the Canadian National Advisory Committee on Immunization’s (NACI) annual Statement on Seasonal Influenza Vaccines for 2024-2025 recommends Fluzone® High-Dose Quadrivalent among the preferential influenza vaccines over standard-dose influenza vaccines.
According to NACI on July 26, 2024, Fluzone® High-Dose Quadrivalent has the most substantial body of supporting evidence among preferentially recommended vaccines for adults 65 years of age and older.
Dr. Angel Chu MD, FRCPC, Infectious disease specialist, Clinical Assistant Professor, University of Calgary, STI Clinic Calgary, and Vice-Chair of Immunize Canada, commented in a press release, “In the newest NACI statement, Fluzone® High-Dose continues to be recommended for adults 65 years of age and older. NACI also recognizes Fluzone® High-Dose has the most substantial body of supporting evidence among flu vaccines for seniors.”
Influenza can cause mild to severe illness. Some populations, especially young children and adults 65 and older, are at a higher risk for serious influenza complications.
Sanofi says vaccination is the most effective way to prevent influenza and its complications.
Earlier this year, the WHO recommended that trivalent vaccines be deployed during the 2024-2025 northern hemisphere influenza season.
On June 27, 2024, the U.S. Centers for Disease Control and Prevention vaccine committee meeting included presentations focused on Considerations and Proposed Recommendations for the 2024-25 Influenza Season in the United States. Physicians, nurses, and pharmacists can offer patients up to nine different influenza vaccines for the 2024 - 2025 flu season.

The Ontario Ministry of Health has announced the first publicly funded universal program with Beyfortus® (nirsevimab) for all newborns and infants born in 2024 and through the 2024-2025 respiratory syncytial virus (RSV) season in the Northern Hemisphere.
Beyfortus single-dose administration can be timed to the start of the RSV season.
RSV is a common respiratory virus that often impacts children and can lead to lung infections such as bronchiolitis and pneumonia.
As of July 25, 2024, this new passive immunization program also includes some high-risk children up to 24 months old.
Beyfortus is administered directly to newborns and infants and offers rapid protection via an antibody without requiring immune system activation.
Delphine Lansac, General Manager, Vaccines, Sanofi Canada. commented in a press release on July 25, 2024, "Today's announcement by the Government of Ontario is a significant milestone. Providing universal access to Beyfortus® to help protect all infants in Ontario means that parents can focus on the joys of a new baby and worry less about experiencing a severe RSV infection."
"This new program builds on our 110-year heritage as a committed partner supporting public health in Canada. Our objective continues to be protecting the health of Canadians with innovative solutions and introducing Beyfortus® is a step forward to protect babies and make a positive difference for families and the healthcare system."
Health Canada issued a Notice of Compliance for Beyfortus in April 2023. Additionally, the single-dose, extended half-life monoclonal antibody was approved by the U.S. FDA in July 2023 and the European Union in October 2022.
According to media reports, access to Beyfortus is expected to meet demand in the United States during the second half of 2024.
AstraZeneca, responsible for Beyfortus manufacturing, confirmed regulatory applications for two additional filling lines have been submitted to health authorities to expand supply. This production expansion is anticipated to augment capacity compared to the one licensed line.

Bavarian Nordic A/S today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) had recommended the approval of a type II variation for IMVANEX® (MVA-BN, JYNNEOS®) smallpox and mpox vaccine.
This EMA recommendation includes real-world effectiveness data from the use of the vaccine during the global 2022 mpox outbreak in the marketing authorization.
In real-world studies, vaccine effectiveness against mpox disease was demonstrated at least 14 days after vaccination, with adjusted vaccine effectiveness estimates ranging from 35% (95% CI, -2-59) to 89% (95% CI, 76-95) after one MVA-BN dose and from 66% (95% CI, 47-78) to 90% (95% CI, 86-92) after two MVA-BN doses.
Furthermore, in a surveillance study, MVA-BN reduced the risks of mpox-related hospitalization.
Compared with unvaccinated mpox patients, the odds of hospitalization were 0.27 (95% CI, 0.08-0.65) after one MVA-BN dose and 0.20 (95% CI, 0.01-0.90) after two MVA-BN doses. The estimated relative risk reduction was 73% after one MVA-BN dose and 80% after two MVA-BN doses.
“The 2022 global mpox outbreak provided an opportunity to assess the effectiveness of our vaccine in at-risk populations across different geographies, both before and after exposure to the mpox virus, and we are pleased to receive the recommendation to include real-life data in our marketing authorization in Europe, which confirm a high effectiveness of up to 90% after two doses of the vaccine as recommended by the authorities. It is furthermore encouraging that data show the vaccine to reduce the risk of hospitalizations significantly, thus confirming our vaccine as an important and versatile tool in the fight against mpox globally,” said Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, in a press release on July 26, 2024.
As of July 2024, the JYNNEOS vaccine is commercially available in the United States.



