Search API
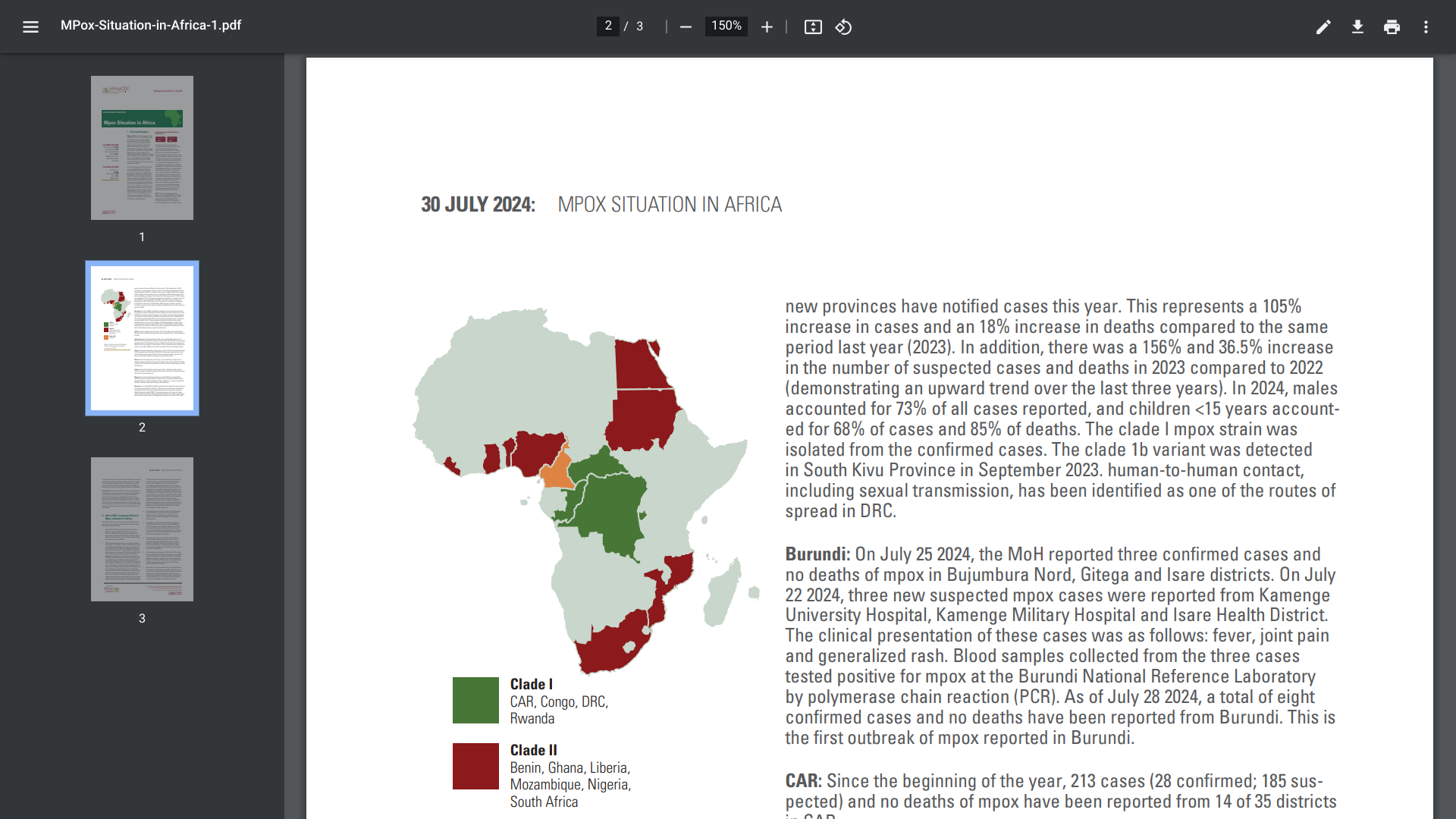
Since the beginning of 2022 and as of late July 2024, a total of 37,583 mpox cases and 1,451 deaths (case fatality rate 3.9%) have been reported from 15 African Union Member States. In an attempt to reduce this outbreak, the U.S. FDA and EMA-approved mpox vaccine is being deployed.
Bavarian Nordic A/S recently announced a new order from the European Health Emergency Preparedness and Response Authority (HERA) for the MVA-BN® (JYNNEOS) vaccine.
HERA will procure 175,420 vaccine doses to donate to the Africa Centres for Disease Control and Prevention (ACDC) to support their strengthened response to the ongoing mpox outbreak in Africa.
Additionally, Bavarian Nordic will donate 40,000 doses to HERA, which will also be donated to the ACDC.
This larger donation follows a recent pledge from Bavarian Nordic for 15,000 doses as part of a coordinated response by Gavi, WHO, and UNICEF in the African region.
“Mpox is spreading at an alarming rate in Africa, calling for further action from the international community. We are proud to support HERA’s contribution of vaccines to the region and are pleased to announce an additional donation from Bavarian Nordic,” said Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, in a press release on August 13, 2024.
Currently, two African countries have granted Emergency Use Authorization for the MVA-BN vaccine.
Seperately, the WHO Director-General issued the following statement on August 14, 2024, "In light of the expanding outbreak in east and central Africa, and the potential for further international spread within and outside Africa, I have convened this Emergency Committee under the International Health Regulations to advise me on whether the outbreak represents a public health emergency of international concern."
"When I declared an end to the previous mpox PHEIC last year (2023), I issued standing recommendations under the IHR, which are due to expire next week. I have decided to extend them for another year to support countries in responding to the chronic risk of mpox."
"Were I to decide, on your advice, that the current situation represents a public health emergency of international concern, I would issue temporary recommendations by the IHR, again on your advice."
The JYNNEOS vaccine is available in the United States at designated clinics and pharmacies.
With one approved chikungunya vaccine already available, the U.S. Food and Drug Administration (FDA) has accepted and granted Priority Review for the Biologics License Application (BLA) for Bavarian Nordic A/S CHIKV VLP, a vaccine candidate for immunization to prevent disease outbreaks caused by chikungunya virus infection in individuals 12 years of age and older.
The Priority Review designation means the FDA aims to complete its review within six months. The FDA has assigned a target action date for the Prescription Drug User Free Act of February 14, 2025.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on August 13, 2024, “The FDA review, along with the ongoing review of our CHIKV VLP vaccine by the European Medicines Agency, represent the first regulatory reviews of a chikungunya vaccine for adolescents, potentially providing a broader usage by populations at risk of this debilitating disease.”
CHIKV VLP is currently also under accelerated assessment review with the EMA, potentially supporting approval of the vaccine by the European Commission in the first half of 2025.
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus (CHIKV). The disease typically presents with acute symptoms, including fever, rash, fatigue, headache, and often severe and incapacitating joint pain.
While mortality is relatively low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Over the past few decades, CHIKV has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Region of the Americas, often causing large, unpredictable outbreaks.
As of August 8, 2024, the Pan American Health Organization (PAHO) reported over 371,167 CHIKV cases in the Americas this year. Between 2013 and 2023, the PAHO reported more than 3.7 million CHIKV cases in the Americas.
The U.S. CDC reported from 2006 to 2023, 4,590 travel-related CHIKV cases were reported in the U.S., in areas such as Florida.
However, Locally acquired cases have not been reported in U.S. states or territories since 2019.
According to a recent Morbidity and Mortality Weekly Report, more than half of school-aged children in American Samoa have evidence of a dengue fever virus infection.
The estimated seroprevalence among all students aged 7–16 years was 59% (95% CI = 47%–71%) and was 60% (95% CI = 48%–72%) among those age-eligible for vaccination (i.e., those aged 9–16 years).
Dengue seroprevalence was lowest among children aged 8 (46%; 95% CI = 32%–60%).
The U.S. CDC's Notes from the Field (73(31);686–688) published on August 8, 2024, suggests American Samoa exceeds the minimum 20% threshold established for the introduction of recommended dengue vaccines to reduce the risk of hospitalization and severe dengue in seronegative children and adolescents.
The authors concluded, "In American Samoa, dengue vaccines could be part of a broader strategy for dengue control."
As of August 13, 2024, access to dengue vaccines in the United States is very limited.
American Samoa is located in the Pacific Ocean, halfway between Hawaii in the north and New Zealand in the south. The CDC recommends checking the vaccines and medicines list and visiting your healthcare provider at least a month before your trip to American Samoa to get necessary supplies.
These authors disclosed no potential conflicts of interest.
Valneva SE today reported its consolidated financial results for the first half of the year, which ended June 30, 2024. On August 13, 2024, Valneva reported total revenues of €70.8 million, Net Profit of €34.0 million, and Cash position of €131.4 million.
Valneva’s commercial portfolio consists of three travel vaccines: IXIARO®/JESPECT®, DUKORAL®, and the recently launched IXCHIQ®, the first monovalent, single-dose, live-attenuated chikungunya vaccine approved by the U.S. Food and Drug Administration.
The Company recently confirmed that IXCHIQ's two-year antibody persistence data were published in the Lancet Infectious Diseases and reported positive six-month data for the Phase 3 adolescent study of IXCHIQ; it expects to submit label extensions for 12 to 17 years in the U.S., Europe, and Canada in the second half of 2024.
In a press release, Peter Bühler, Valneva’s Chief Financial Officer, commented, “Our first half sales performance is in line with our expectations."
"We aim to further capitalize on the travel industry recovery as we focus on ramping up sales for IXCHIQ® to support our commercial growth while continuing to execute on our key R&D and regulatory milestones."
The Company also distributes certain third-party products in countries where it operates its own marketing and sales infrastructure.
For example, the Company has an exclusive worldwide license for the S4V Shigella vaccine candidate, adding an attractive Phase 2 clinical asset to Valneva’s R&D pipeline without impacting full-year or mid-term financial guidance.
The half-year financial report, including the condensed consolidated interim financial report and the half-year management report, is available on the Company’s website (Financial Reports—Valneva).
On August 13, 2024, Valneva CEO Thomas Lingelbach spoke on YouTube about the Company’s half-year results and plans for the rest of 2024.

Pfizer Inc. today announced positive top-line safety and immunogenicity results from substudy B of the ongoing pivotal Phase 3 clinical trial.
The trial is evaluating two doses of the ABRYSVO™ vaccine in immunocompromised adults aged 18 and older at risk of developing severe respiratory syncytial virus (RSV)- associated lower respiratory tract disease.
ABRYSVO was well-tolerated during the trial, showing a safety profile consistent with findings from other vaccine studies.
While the company evaluated two doses, a single 120 µg dose of ABRYSVO generated a strong neutralizing response against both subtypes of RSV, RSV-A, and RSV-B across all cohorts and age groups in the study.
Pfizer plans to share these findings at an upcoming scientific conference, publish them in a peer-reviewed scientific journal, and submit the data to the regulatory agencies for review.
“Immunocompromised adults, such as patients with cancer or autoimmune disorders, have a substantially increased risk of experiencing severe complications from RSV, yet there are currently no vaccines approved for those aged 18 to 59 in the U.S.,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on August 12, 2024.
“We are encouraged by the positive top-line data from this study, which provide important evidence that ABRYSVO has the potential to address a significant unmet need in this vulnerable population.”
These most recent data in immunocompromised adults build on the body of evidence supporting the profile of ABRYSVO in high-risk adults.
As of early August 2024, three RSV vaccines have been approved for use in the U.S., and several vaccine candidates are conducting late-stage studies.
Additionally, the U.S. CDC reported on August 9, 2024, that RSV sections were generally low, except for the state of Florida.