Search API
The United States Agency for International Development (USAID) announced up to an additional $35 million in emergency health assistance to bolster response efforts for the clade Ib mpox outbreak in Central and Eastern Africa, pending U.S. Congressional Notification.
This new commitment on August 20, 2024, brings the total U.S. government support for the affected countries in the region to more than $55 million in response to the ongoing mpox outbreak.
USAID support includes assistance with surveillance, diagnostics, risk communication, community engagement, infection prevention and control, case management, and vaccination planning and coordination.
The USAID support includes donating 50,000 doses of the third-generation JYNNEOS® (MVA-BN®, IMVAMUNE®) mpox / smallpox vaccine to the Democratic Republic of the Congo (DRC), the country most severely impacted by the outbreak.
Since 2023, this mpox outbreak has extended beyond the DRC, with several other countries in the region reporting cases in 2024, including countries where mpox has historically not been reported.
The current mpox outbreak differs in disease severity from the global clade II outbreak that began in May 2022, impacting the United States.
According to real-world evidence published in The Lancet Infectious Diseases today, this analysis is the first to provide estimates of Merck's Ervebo® (rVSV-ZEBOV) vaccine against Zaire Ebolavirus disease amid the widespread use of the vaccine during a large outbreak.
Announced on August 20, 2024, these findings confirm that Ervebo is highly protective against 84% (95% credible interval, 70% to 92%) of Ebolavirus disease and supports its use during outbreaks, even in challenging contexts such as in the eastern Democratic Republic of the Congo (DRC).
This finding is essential since Ebolaviruses are endemic in the DRC.
In a related Editorial, the authors wrote the 2018–20 Ebola virus disease epidemic in the DRC resulted in 3,470 reported cases and remains the second-largest Ebolavirus outbreak in recorded history worldwide. The initial Ebola outbreak was in 1976.
In November 2019, the World Health Organization prequalified the Ervebo vaccine. The U.S. Food and Drug Administration approved it on December 19, 2019.
Médecins Sans Frontières (Doctors Without Borders) funded this study.
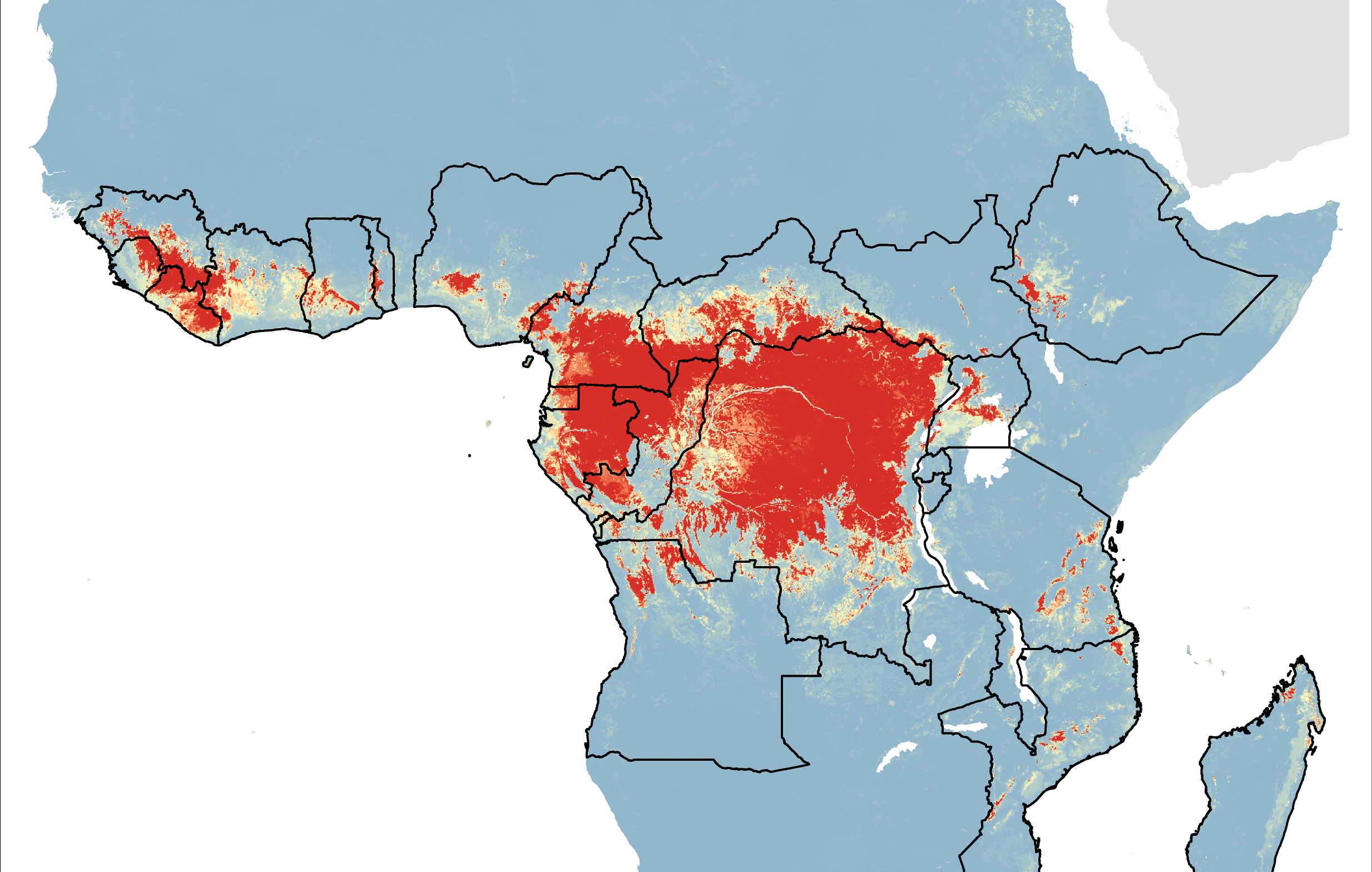
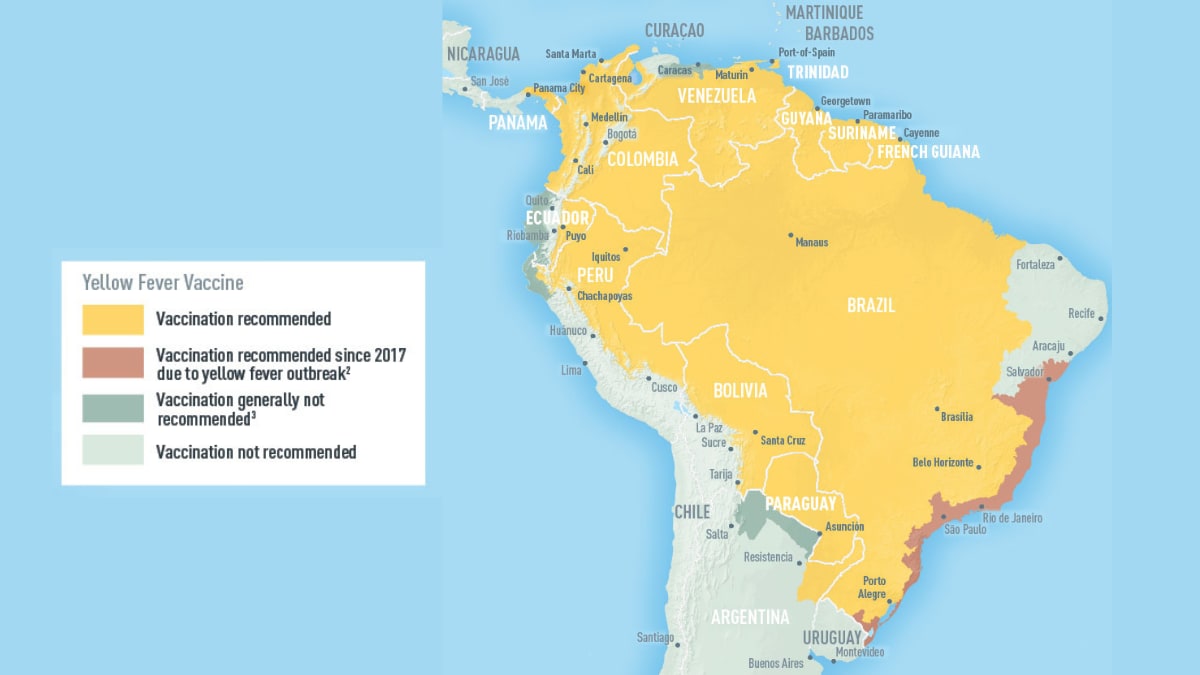
In 2024, yellow fever outbreaks remain a health threat in tropical regions of Africa and South America. The good news is that vaccines have been proven safe and effective for protecting international travelers visiting these areas.
However, new yellow fever vaccines with improved production scalability and enhanced efficacy are needed to reduce outbreaks.
The Lancet Infectious Diseases recently published results from a first-in-human phase 1 study on the safety and immunogenicity of a new Vero cell line-derived yellow fever vaccine, vYF-247.
Produced by Sanofi, the vYF-247 vaccine showed similar safety and immunogenicity to the U.S. FDA-approved YF-VAX vaccine.
These researchers concluded that the vYF-247 vaccine with a 5 Log CCID50 dose showed optimal viremia, safety, and immunogenicity and was chosen for further development.
Until a new vaccine is approved, the YF-VAX® vaccine remains available at travel clinics and pharmacies in the United States. For those travelers who were already vaccinated, the U.S. CDC says yellow fever vaccine booster doses are unnecessary.
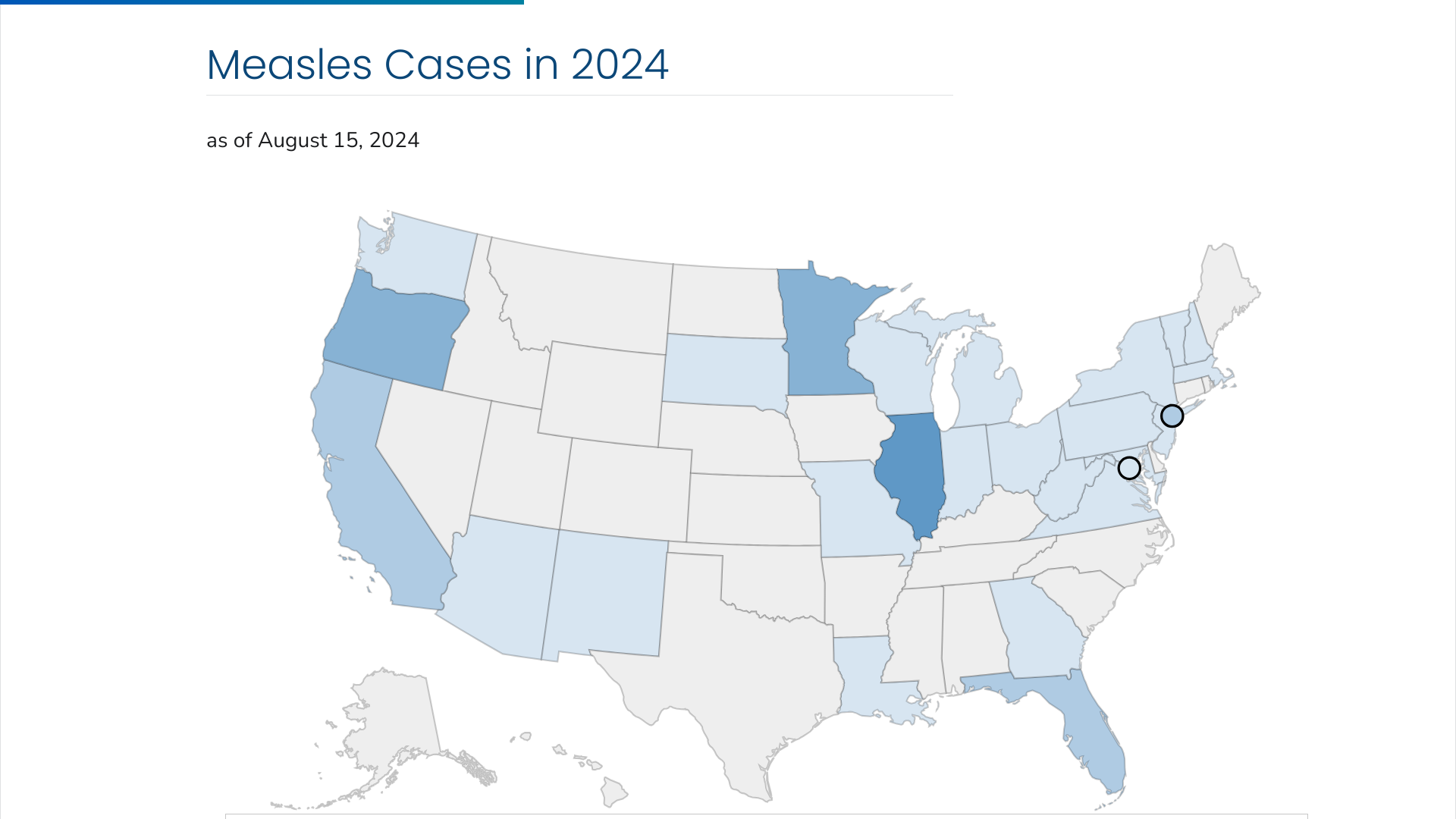
The Oregon Health Authority (OHA) has reported four new measles cases in 2024, bringing the total to 30 across three counties. Marion County has the most cases, followed by Clackamas County and Multnomah County.
As of August 21, 2024, all measles patients were unvaccinated, and twelve were younger than ten.
These counties and OHA have been sharing information with the public so “we can let members of the public know they may have been exposed to measles,” Clackamas County Health Officer Sarah Present, M.D. said in a recent press release.
Dr. Present noted that since measles is so contagious, an estimated 96% of the population needs to have received two doses of measles vaccine to protect the community's most vulnerable members via community or “herd” immunity.
In Oregon, measles vaccines are available at health clinics and local pharmacies.
As of August 15, 2024, the U.S. CDC confirmed that 27 U.S. jurisdictions reported 219 measles cases this year. In 2023, 20 jurisdictions reported 59 measles cases for the entire year.
Emergent BioSolutions today announced it has pledged to donate 50,000 doses of its ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) through a humanitarian relief organization to the Democratic Republic of the Congo (DRC) and the other impacted countries of Burundi, Kenya, Rwanda, and Uganda.
In October 2023, Emergent filed a supplemental Biologics License Application to the U.S. FDA seeking an expanded indication for the ACAM2000 vaccine to include immunization against the mpox virus. The FDA target for review completion in the third quarter of 2024.
These efforts are in response to the WHO’s recent statement declaring that the upsurge of mpox clade 1 in African countries constitutes a public health emergency of international concern under the International Health Regulations.
“Africa CDC estimated they will need 10 million doses to control the epidemic in the continent,” said Dr. Raina McIntyre, Professor of Global Biosecurity, NHMRC L3 Research Fellow, Head, Biosecurity Program, Kirby Institute, University of New South Wales Sydney, in a press release on August 19, 2024.
“It is unlikely there will be enough supply of 3rd generation vaccines (JYNNEOS®, MVA-BN®) to control the epidemic in Africa, given demand in other countries.”
A recent study found that the Fluad® MF59-adjuvanted influenza vaccine (aTIV) was more effective than the high-dose flu vaccine (HD-TIV) at preventing severe respiratory complications in older adults with risk factors.
Published in Open Forum Infectious Diseases on August 16, 2024, the study included 1,115,725 aTIV and 2,561,718 HD-TIV recipients. For the primary outcome, the analysis found comparable effectiveness between aTIV and HD-TIV (rVE [95% CI]: 5.2% [-5.9–15.1]) among those with 0 risk factors, whereas aTIV was more effective than HD-TIV among patients with ≥1, 1–2, or ≥3 risk factors (12.5% (10.0–15.0), 18.4% (13.7–22.9), and 10.4% (7.4–13.3), respectively).
The same trends were observed for the secondary outcomes.
Previous studies have found the two vaccines to be similar in effectiveness in older adults.
The Fluad vaccine has an extensive clinical legacy and has been licensed in 30 countries since its first approval in 1997. Fluad is available at most pharmacies in the U.S. for the 2024-2025 flu season.

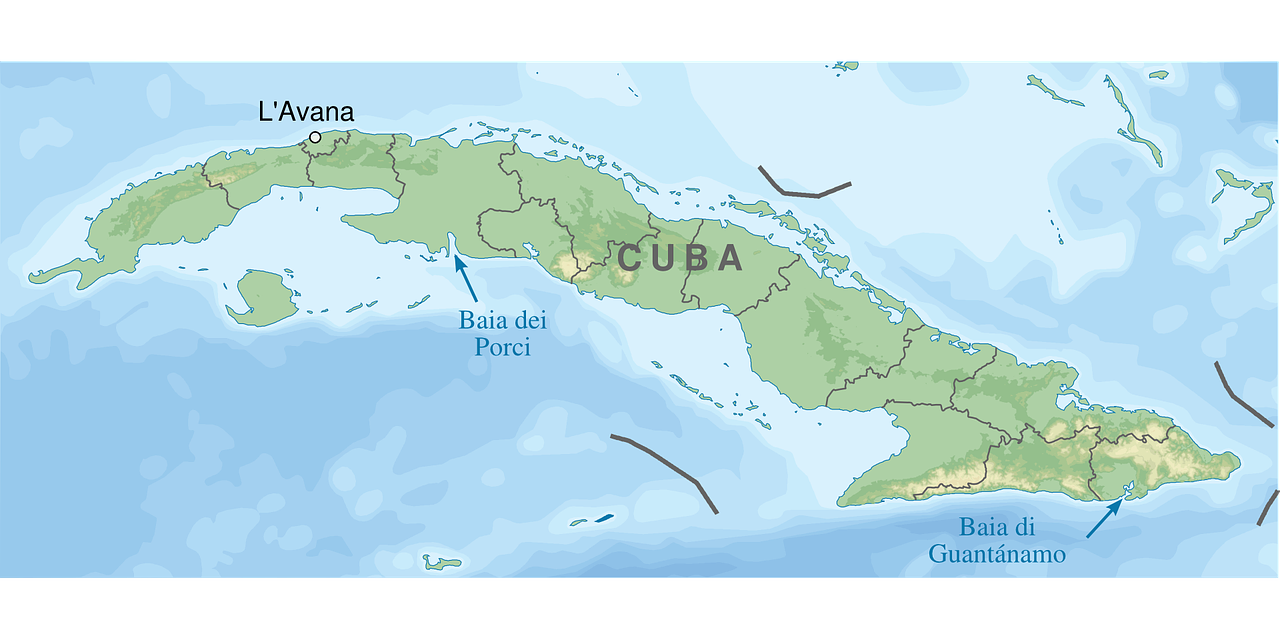
Throughout 2024, Cuba has been grappling with an outbreak of Oropouche Fever. And now, its western neighbor, the United States, has started to report cases related to travelers from Cuba.
The Florida Department of Health (FDH) has recently reported 11 Oropouche Fever cases.
As of August 10, 2024, these Florida cases had their onset in 2024 and were found in individuals who had traveled to Cuba two weeks before showing symptoms.
The Oropouche reported cases were found in the following Florida counties: Hillsborough (4), Lee (2), Miami-Dade (1), Orange (2), and Polk (2).
Throughout 2024, more than 8,000 Oropouche cases, including two deaths and five cases of vertical transmission, were reported by the U.S. CDC.
According to the CDC, approximately 60% of people infected with the Oropouche virus become symptomatic. The incubation period is typically 3–10 days. Although people exposed to biting midges or mosquitoes infected with the virus are most at risk for developing the disease, the risk factors for more severe Oropouche virus are not well-defined.
The initial clinical presentation is similar to diseases caused by dengue, Zika, and chikungunya viruses.
In the U.S., healthcare providers should contact local health departments to facilitate diagnostic testing.
As of August 19, 2024, no approved Oropouche vaccines are available.
In addition to Oropouche cases, FDH reported 18 locally acquired dengue fever virus cases and numerous travel-related dengue cases as of week #32.