Search API
Bavarian Nordic A/S recently announced it received a new contract of 440,000 doses to supply its MVA-BN® smallpox and mpox vaccine from an undisclosed European country.
The Company confirmed all vaccines under this contract will be delivered in 2024.
Immediate access to these vaccines is essential since the mpox clade 1b outbreak is spreading in Africa.
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on August 21, 2024, “Bavarian Nordic can still supply up to 10 million doses of our smallpox and mpox vaccine by the end of next year, with 2 million doses of this capacity available during the remaining part of this year.”
The MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic (JYNNEOS®, IMVANEX®, and IMVAMUNE®) is a non-replicating vaccine approved by several countries, including the United States.

Moderna, Inc. announced that the European Commission (EC) has granted marketing authorization for mRESVIA®, an mRNA respiratory syncytial virus (RSV) vaccine.
RSV is a highly contagious seasonal respiratory virus that causes an exceptionally high burden of disease in infants and older adults.
As of August 23, 2024, this EC authorization is indicated to protect adults aged 60 years and older from lower respiratory tract disease caused by RSV infection.
The marketing authorization is valid in all 27 EU member states, as well as Iceland, Liechtenstein, and Norway.
"The EC's approval of mRESVIA is an important milestone for public health and highlights Moderna's mRNA leadership," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.
In the European Union, RSV is estimated to cause approximately 160,000 hospital admissions in adults each year, with 92% of these admissions occurring in adults aged 65 and over.
In the United States, the RSV season has already begun in Florida and is expected to spread throughout the U.S.
For the 2024-2025 RSV season in the U.S., three vaccines and one monoclonal antibody were approved by the U.S. FDA.
Novavax Inc. today announced, 'We are working productively with the U.S. Food and Drug Administration (FDA) as they complete their review, including providing additional information as needed, and the FDA has committed to moving swiftly on regulatory authorization.'
'We expect to have authorization in time for peak vaccination season.'
Novavax filed for U.S. Emergency Use Authorization of our 2024-2025 formula protein-based COVID-19 vaccine (NVX-CoV2705) in June 2024.
In the United States, Novavax's products have been and will be available after FDA authorization in thousands of locations nationwide, including pharmacies.
For example, Walgreens recently confirmed their pharmacists are available to help patients navigate the latest vaccination guidance, including the timing of vaccinations, given the uneven geographical spreading of the coronavirus.
Additionally, numerous countries have authorized Novavax's COVID-19 vaccines over the past few years.
Novavax wrote on August 22, 2024, 'Our 2024-2025 formula COVID-19 vaccine targets JN.1, the "parent strain” of currently circulating variants and should provide acceptable coverage and cross-reactivity against JN.1 lineage viruses, including KP.2.3, KP.3, KP.3.1.1 and LB.1.1.'
'Upon authorization, Novavax’s vaccine will be the only protein-based option available in the U.S. for individuals aged 12 and older to prevent COVID-19.'
On August 8, 2024, Novavax reported it achieved total revenue of $415 million in the second quarter of 2024 and ended the period with $1.1 billion in Cash.
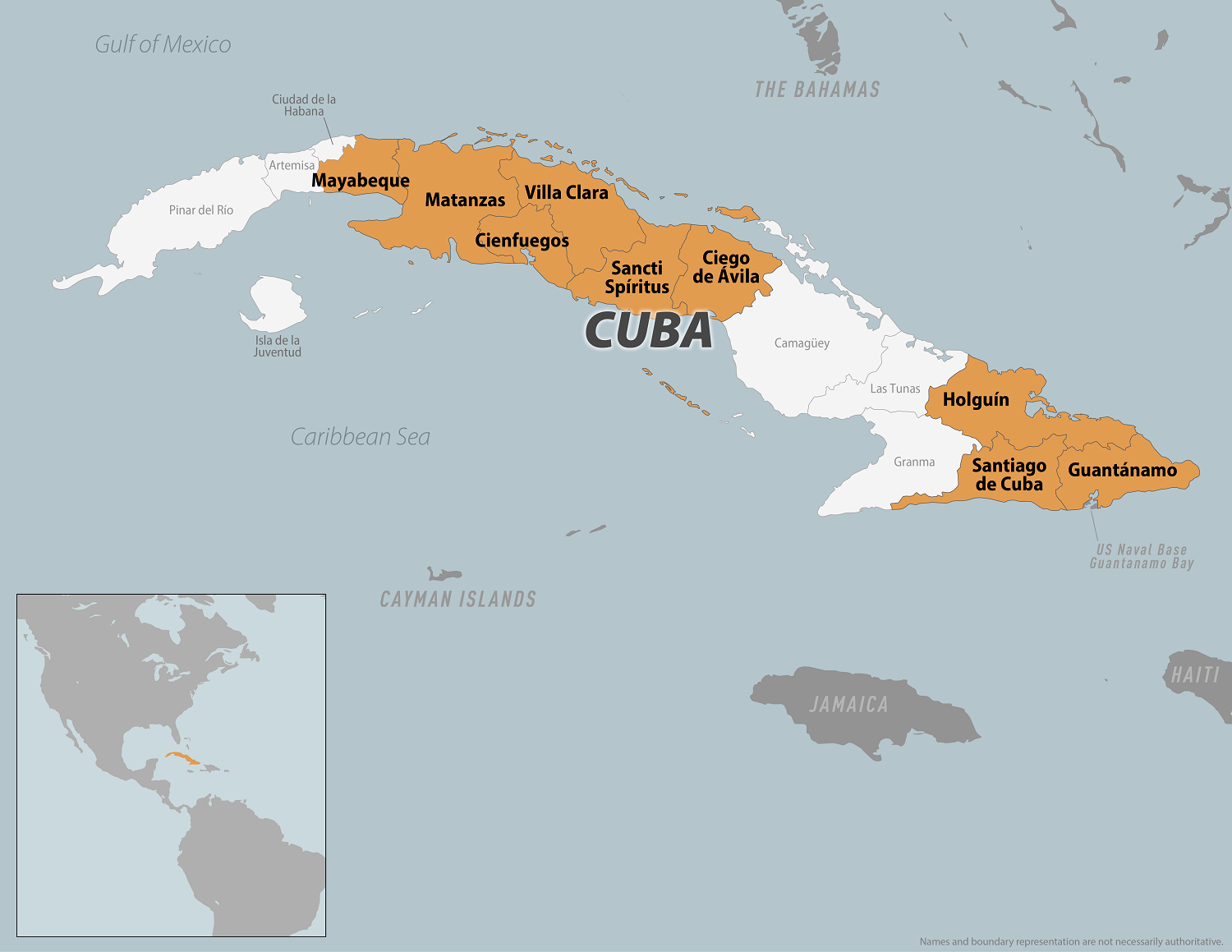
Cuba's Minister of Public Health, José Ángel Portal Miranda, recently announced over 400 Oropouche virus disease cases have been confirmed on the island since late May 2024.
According to Cuba Headlines reporting, Cuba ranks second in the Region of the Americas for the number of infections behind Brazil (7,284).
Infected biting midges and some mosquitoes are spreading the virus.
Currently, there are no efficient vector control measures for the Culicoides paranesis.
As of August 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) says there is no evidence of local transmission of Oropouche virus disease in the United States. However, various states, such as Florida (12), report travel-related cases.
The virus was first detected in 1955 in Trinidad and Tobago near the Oropouche River. Since then, outbreaks of the Oropouche virus have been reported in Bolivia, Brazil, Colombia, Ecuador, French Guiana, Panama, and Peru.
The incubation period for Oropouche virus disease is 3–10 days, says the U.S. CDC. Typically, the disease starts with the abrupt onset of fever (38-40°C), followed by a headache, chills, myalgia, and arthralgia.
People typically recover without long-term sequelae. However, there have been a few deaths reported and vertical transmission of Oropouche virus causing fetal deaths and congenital abnormalities.
The best way to protect themselves from Oropouche is to prevent bites from biting midges and mosquitoes.
According to the CDC's Level 2 Travel Health Advisory, updated on August 15, 2024, travelers to Cuba should prevent bug bites during visits to protect themselves from infection, as there are no vaccines to prevent Oropouche virus disease.
Note - Headlines was edited on Aug, 24, 2024,
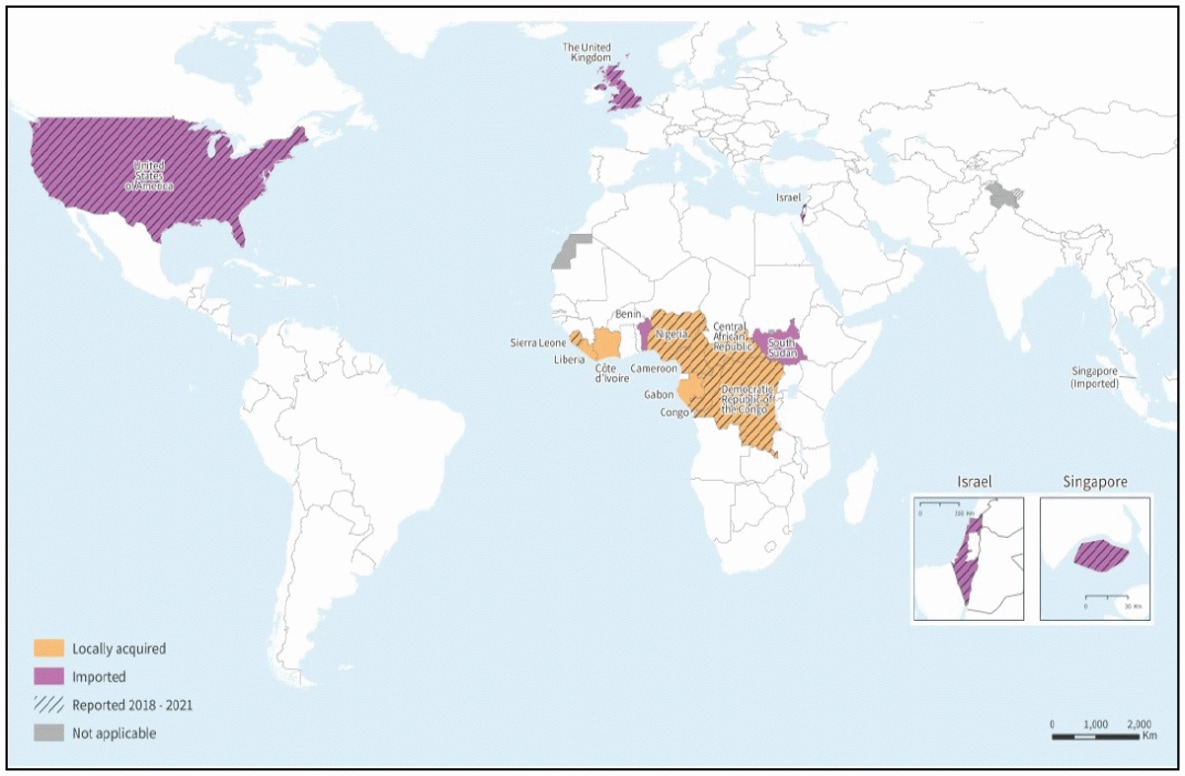
The World Health Organization (WHO) announced today that three vaccines are available to prevent mpox in different countries.
Published on August 22, 2024, the WHO's Disease Outbreak News confirmed the MVA-BN® (JYNNEOS®, IMVAMUNE®), LC16-KMB, and OrthopoxVac are available in certain countries. However, OrthopoxVac has not yet been commercialized.
Based on extensive clinical research, the WHO recommends using MVA-BN or LC16 vaccines when the others are not available.
While the ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections, the WHO does not recommend it.
Furthermore, mpox vaccination is recommended by WHO and the U.S. CDC for individuals at high risk of exposure, such as when visiting mpox outbreak areas.
Takeda Canada Inc. today annonced a new report, Enhancing Diagnosis, Access, Care, and Treatment, highlighting the urgent need for innovative funding models and collaboration to help accelerate Canada’s National Strategy for Drugs for Rare Diseases.
Nearly 200 novel drugs for rare diseases are being developed and are expected to launch in Canada within the next ten years. It’s estimated only 5% of rare diseases have an approved treatment.
A “rare” disease is any disease that affects a minimal number of individuals. It is often genetic, chronic throughout a patient’s life, and life-threatening. With rare diseases affecting relatively limited patients, innovative treatments are often unavailable.
The impact of rare diseases is significant, with approximately one in 12 Canadians, two-thirds of whom are children.
“Canadians living with rare diseases have every reason to be optimistic,” says Durhane Wong-Rieger, President & CEO of the Canadian Organization for Rare Disorders, in a press release on August 22, 2024.
“Hundreds of new therapies are being developed, many targeting the 95% of rare diseases with no known treatment! We must leverage the $1.5 billion Rare Disease Drug Strategy,
The journey toward appropriately managing a rare disease is long and challenging. On average, it takes 6 to 8 years before a patient receives a correct diagnosis; this time, they will see an average of eight physicians and receive two to three misdiagnoses.
Takeda also produces innovative products, such as QDENGA®, an approved two-dose vaccine that prevents dengue fever and/or severe dengue in adults caused by any of the four serotypes of the dengue virus.
This dengue vaccine is authorized in about 40 countries and does not require pre-admission testing.




