Search API
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended marketing authorization for the companies’ Omicron KP.2-adapted monovalent COVID-19 vaccine (COMIRNATY ® KP.2) for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals six months of age and older.
Pfizer Inc. and BioNTech SE announced that the European Commission will review the CHMP’s recommendation and is expected to make a final decision soon.
The CHMP recommendation dated September 19, 2024, is based on the non-clinical and manufacturing data of the Omicron KP.2-adapted vaccine and the clinical and real-world evidence supporting the safety and efficacy of prior formulas of the COVID-19 vaccines by Pfizer and BioNTech.
Pending the EC's authorization of the Omicron KP.2-adapted vaccine, both the Omicron KP.2-adapted vaccine and the Omicron JN.1-adapted vaccine will be available across the EU, though availability will vary based on individual country government requests and national recommendations.

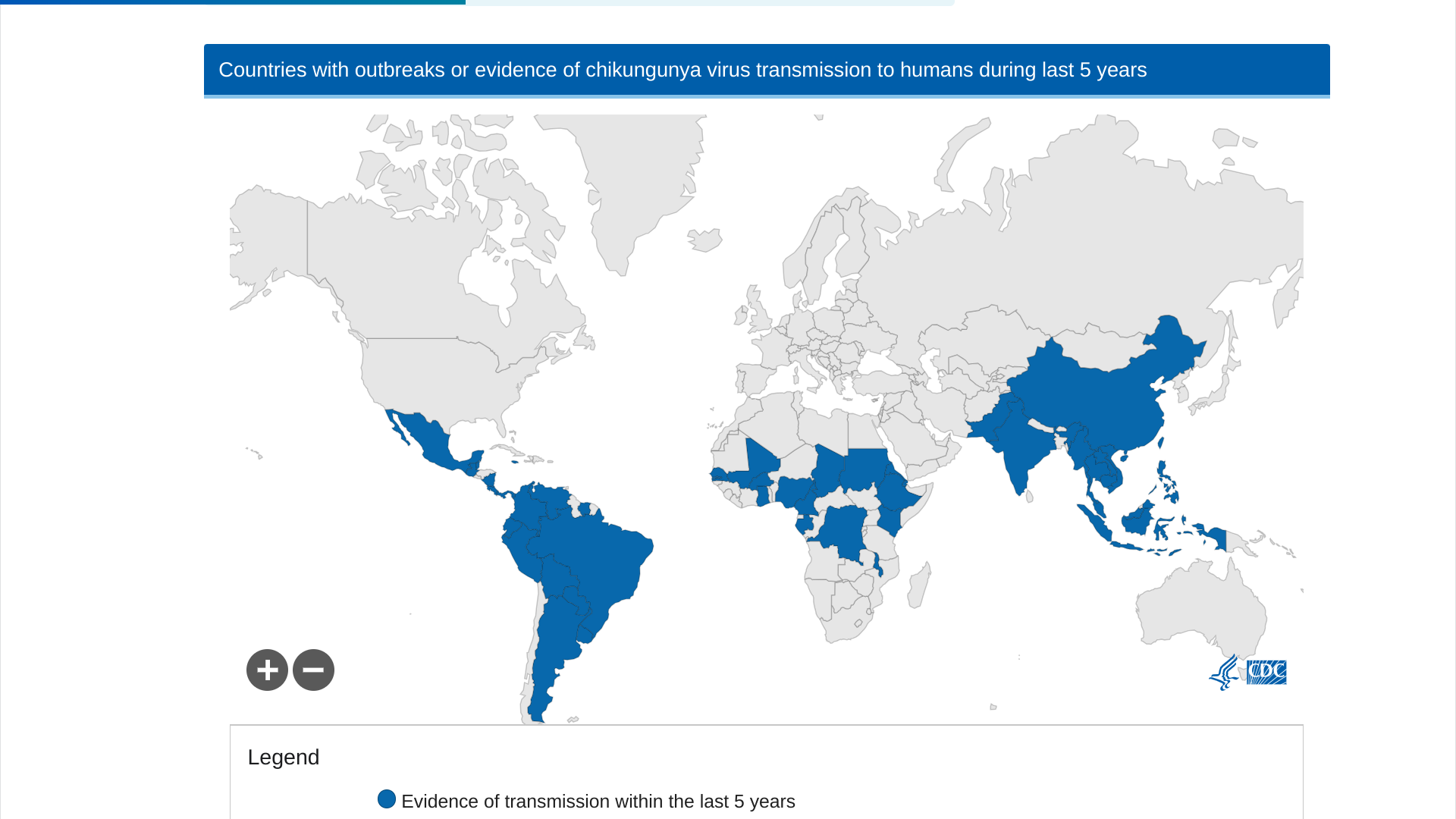
The peer-reviewed journal The Lancet Infectious Diseases recently published interim results of a double-blind, randomized, placebo-controlled phase 3 trial in adolescents of the U.S. FDA-approved, single-dose IXCHIQ® (VLA1553) chikungunya vaccine.
In an article published on September 4, 2024, these researchers concluded that VLA1553 was generally safe and induced seroprotective titers in almost all vaccinated adolescents, with favorable safety data in seropositive adolescents at baseline.
VLA1553 induced seroprotective chikungunya virus neutralizing antibody levels in 247 of 250 (98.8%, 95% CI 96·5–99·8) participants 28 days after vaccination.
This data supports using VLA1553 to prevent diseases caused by the chikungunya virus among adolescents and in endemic areas in the Region of the Americas.
As of September 22, 2024, the Pan American Health Organization reported 390,669 CHIKV cases. Specifically, Brazil has confirmed 170 related deaths this year.
If you are traveling to an area at risk for chikungunya, the U.S. CDC suggests discussing vaccination options with your healthcare provider at least one month before departing abroad.
The U.S. Department of State published an updated Level 2: Exercise Increased Caution advisory for visitors to the Kingdom of Denmark, Greenland, and the Faroe Islands.
About 63 million international and domestic tourists visited Denmark in 2023, including over 600,000 from the United States.
As of September 17, 2024, the State Department advises travelers to be aware of their surroundings when traveling to tourist locations and crowded public venues. Additionally, travelers should enroll in the Smart Traveler Enrollment Program to receive alerts, which makes locating you in an emergency easier.
Furthermore, Denmark has excellent medical facilities, such as modern and fully equipped hospitals. However, medical facilities in Greenland and the Faroe Islands are limited, and evacuation is required for serious illness or injury. For emergency services in the Kingdom of Denmark, dial 112, and vaccine information is posted at this link.
The U.S. CDC says those travelers who may be at increased risk of an infectious disease due to their work, lifestyle, or underlying health problems should be up to date with recommended vaccines, which may need to be administered one month before visiting Denmark.
Health clinics and pharmacies in the U.S. generally offer travel vaccines.

The Minnesota Department of Health recently affirmed the greater Twin Cities area is experiencing an ongoing outbreak of measles cases, with the virus spreading mainly among unvaccinated children.
The Minneapolis—St. Paul's measles outbreak began in May 2024, and as of September 19, 2024, 51 cases had been confirmed.
Earlier this year, Chicago, Illinois, reported a more significant measles outbreak that impacted 64 people.
Overall, the U.S. CDC has reported 262 measles cases in 32 jurisdictions in 2024.
From a global health-risk perspective, the CDC has issued travel advisories for over 50 countries this year.
Measles is a vaccine-preventable disease. Vaccination is generally recommended for most people, and various vaccines are available at clinics and pharmacies in the U.S.

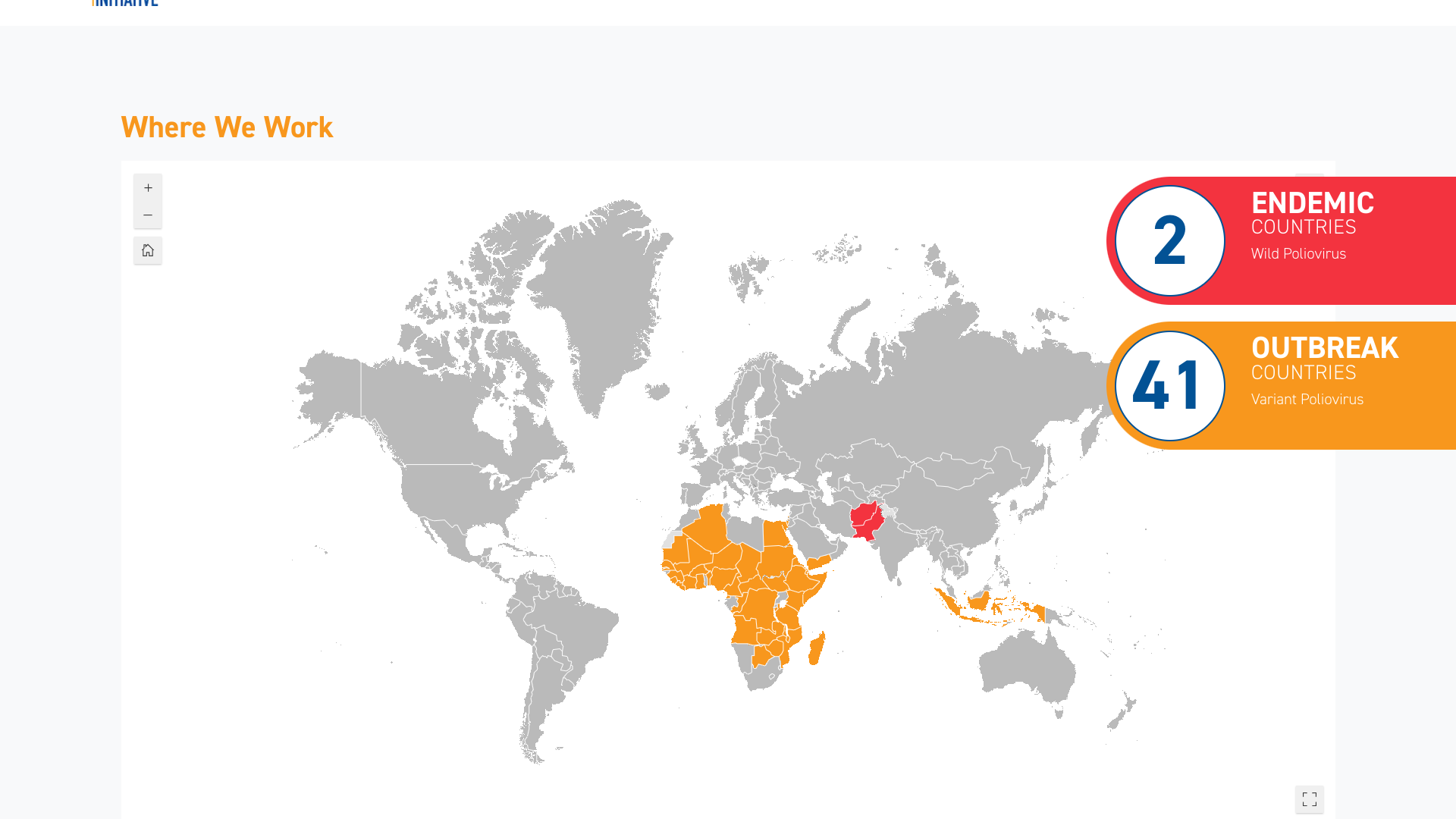
According to the weekly update of the Global Polio Eradication Initiative (GPEI), three countries recently reported new polio cases.
In 2024, Afghanistan reported 19 cases of wild poliovirus type 1 in Kandahar province.
Cameroon reported its first two cases of circulating vaccine-derived poliovirus type 2 (cVDPV2) from Est province this year.
In Nigeria, 53 cases of cVDPV2 were reported from Yobe province, with four new cases added recently.
Overall, two polio-endemic countries are reporting wild polio, Afghanistan and Pakistan, and 41 other countries reporting variant poliovirus.
As of September 21, 2024, the GPEI deploys two types of polio vaccines to stop virus transmission: inactivated polio vaccine (IPV) and oral polio vaccine (OPV). Developing these vaccines to prevent paralytic polio was one of the major medical breakthroughs of the 20th century.
In the United States, the IPV has been offered since 2000.
When planning a visit to a polio-risk country, the U.S. CDC suggests speaking with a travel vaccine expert at least one month before departure about prevention options.
The U.S. Food and Drug Administration (FDA) today announced it approved the nasal influenza vaccine FluMist® for self- or caregiver-administration by adults. This innovative flu shot is sprayed into the nose and has been used safely and effectively for many years.
It is the first influenza vaccine that does not need to be administered by a healthcare provider.
FluMist has been FDA-approved for preventing influenza disease caused by influenza virus subtypes A and B in individuals 2 through 49 years of age for two decades. It was initially approved by the FDA in 2003. In 2007, the FDA approved using FluMist to include children 2 through 5.
Iskra Reic, Executive Vice President of Vaccines and Immune Therapies, AstraZeneca, said in a press release, “The approval of FluMist for self-administration is an important step forward in making vaccines more accessible to fight the high annual burden of influenza."
The vaccine may be administered by a health care provider in a health care setting (including a pharmacy) or by the vaccine recipient or a caregiver who is 18 years of age or older. Vaccine recipients and caregivers who administer FluMist will receive the vaccine, the Prescribing Information, Information for Patients and their Caregivers, and Instructions for Use.
“Today’s approval of the first influenza vaccine for self- or caregiver administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility, and accessibility for individuals and families,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in a press release on September 20, 2024.
FluMist remains available at various clinics and pharmacies for the 2024-2025 flu season.
AstraZeneca markets FluMist® under the Fluenz® Tetra brand internationally.

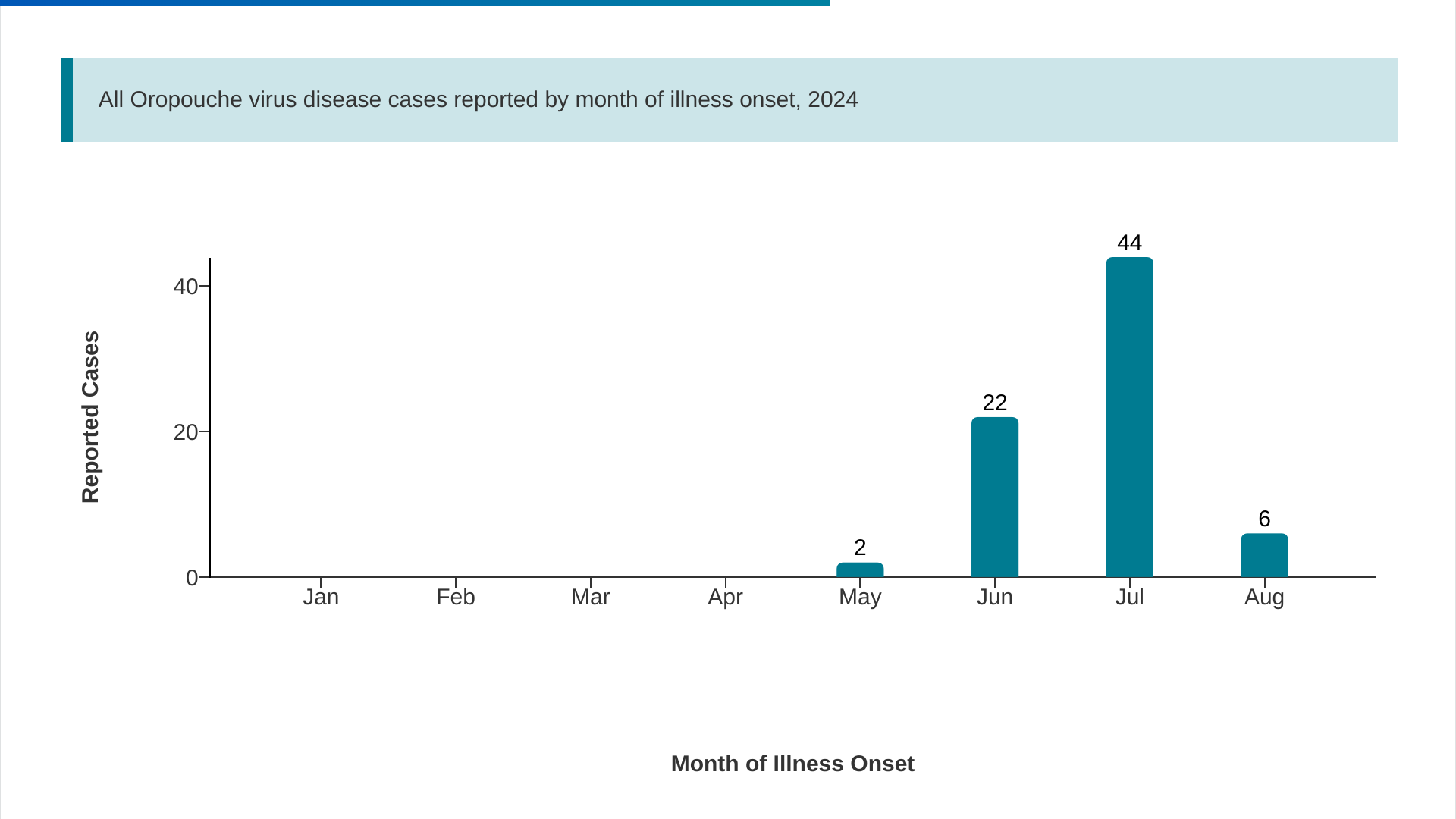
The U.S. Centers for Disease Control and Prevention (CDC) recently reported 22 additional imported cases of the Oropouche virus.
As of September 17, 2024, the total number of cases reported by five U.S. states to 74.
The state of Florida confirmed this week that eleven counties have reported 70 Oropouche cases involving travel to Cuba, which has reported over 500 cases.
The Miami-Dade area (28) has led Florida in reporting cases. The good news is the weekly trends have been decreasing.
In the Region of the Americas, 9,852 cases were confirmed in 2024.
Brazil remains the most affected country, with 7,931 cases and two deaths. Other currently affected countries include Bolivia, Peru, and recently, the Dominican Republic.
The virus is primarily transmitted through the bite of infected midges, small insects that usually bite during the day and inhabit humid areas with organic matter and in forested areas.
Regarding mother-to-child transmission, a total of one fetal death and one case of congenital anomaly have been confirmed in Brazil.
The CDC has confirmed there are not approved vaccines targeting Oropouche virus infections.