Search API
The New Jersey Department of Health (NJDOH) issued an alert regarding a Monmouth County resident who developed measles following recent international travel and visited several locations while infected, including the Jersey Shore University Medical Center in Neptune City.
The NJDOH is working in collaboration with local health officials to identify and notify people who might have been exposed during the time the individual was infectious.
As of October 11, 2024, no additional N.J. cases have been identified. However, secondary measles cases would be expected to occur no later than October 29, 2024.
This is the third confirmed case of measles reported in N.J. and the 267th in the United States this year.
The Department urges all New Jersey residents planning to travel, regardless of destination, to ensure they are current on all routine and travel vaccinations, especially MMR vaccinations. Measles vaccines are offered at clinics and pharmacies in 2024.
On September 24, 2024, the U.S. CDC republished a global Travel Health Notice identifying measles outbreaks in 57 countries.
Dengue is a mosquito-borne viral infection rapidly emerging as a pandemic-prone viral disease across the globe. The World Health Organization (WHO) says the incidence of dengue has increased 30-fold over the last 50 years, especially during rainy seasons.
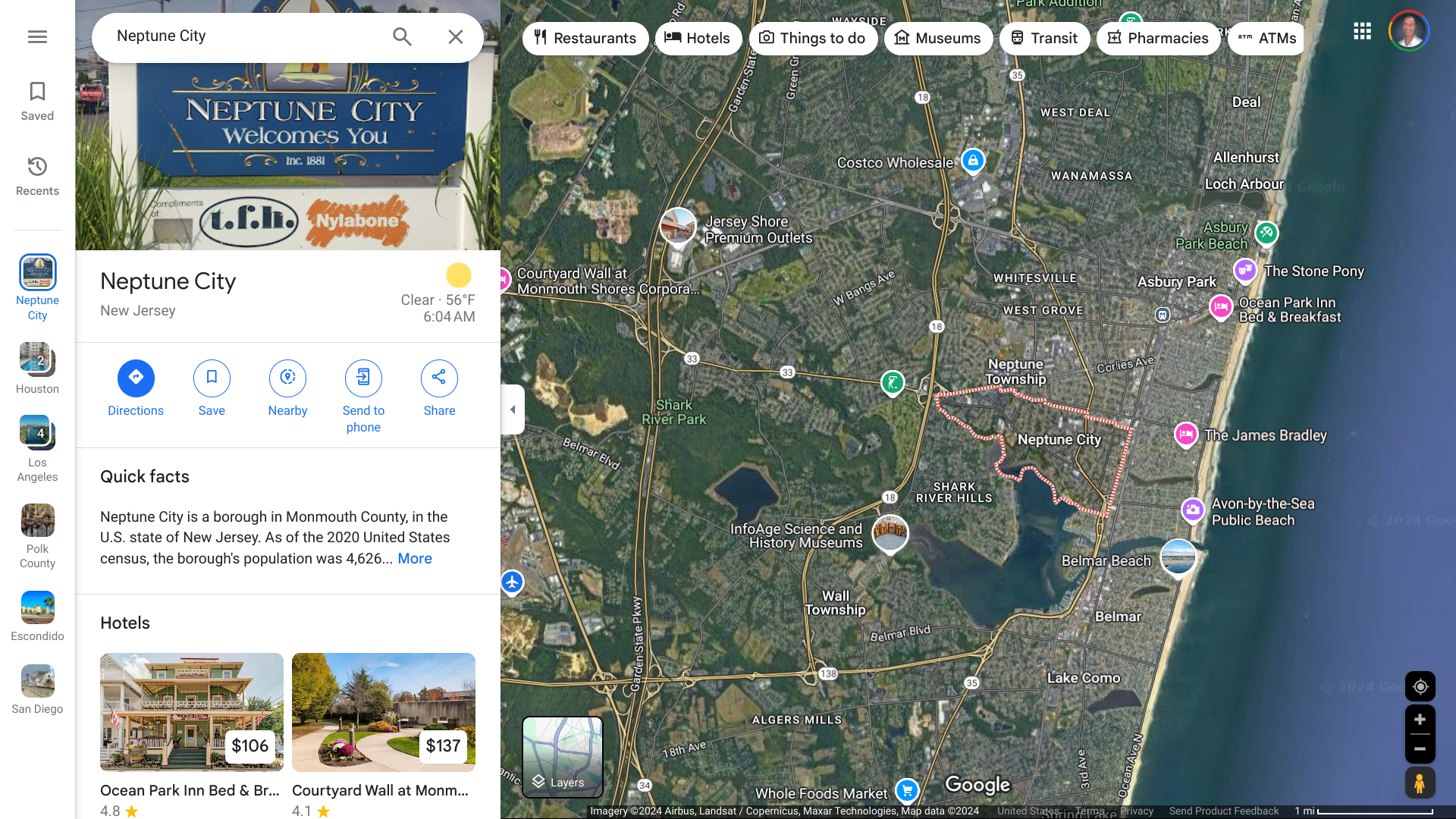
In 2023, over 500,000 dengue cases and 750 deaths were reported from eight countries/territories/areas in the WHO Western Pacific Region, which includes Vietnam.
The WHO reported on October 3, 2024, Vietnam confirmed 76,838 dengue cases, including 12 deaths this year.
According to local news published on September 21, 2024, Vietnam launched its dengue vaccination program in mid-September 2024.
In a media article, Dr. Bach Thi Chinh, Medical Director of VNVC Vaccination System, said that the Ministry of Health approved Takeda's QDENGA dengue vaccine in May 2024 for children from 4 years old and adults.
'The vaccine is particularly effective in preventing reinfection in individuals who have previously contracted dengue fever, which is crucial for Vietnam due to the high prevalence of such cases. Subsequent infections are often more severe than initial ones. Therefore, timely vaccination is essential for safeguarding patients' health and lives.'
This second-generation dengue vaccine will help Vietnam reduce the disease burden and minimize the number of hospitalizations.
Takeda's dengue vaccine is offered in about 40 countries in 2024.
In a rebuttal to recent U.S. government policy, the World Health Organization (WHO) stated, 'At this time, travel and trade restrictions are ineffective and unnecessary for the control of the ongoing outbreak of Marburg virus disease (MVD) in the Republic of Rwanda and are potentially harmful to the affected societies and economies.'
'In addition, travel and trade restrictions may act as a disincentive for rapidly sharing public health data and information with and amongst the global health community, which is critical for informed outbreak response.
The U.S. CDC stated on October 7, 2024, 'Reconsider nonessential travel to Rwanda, which is experiencing an outbreak of MVD.'
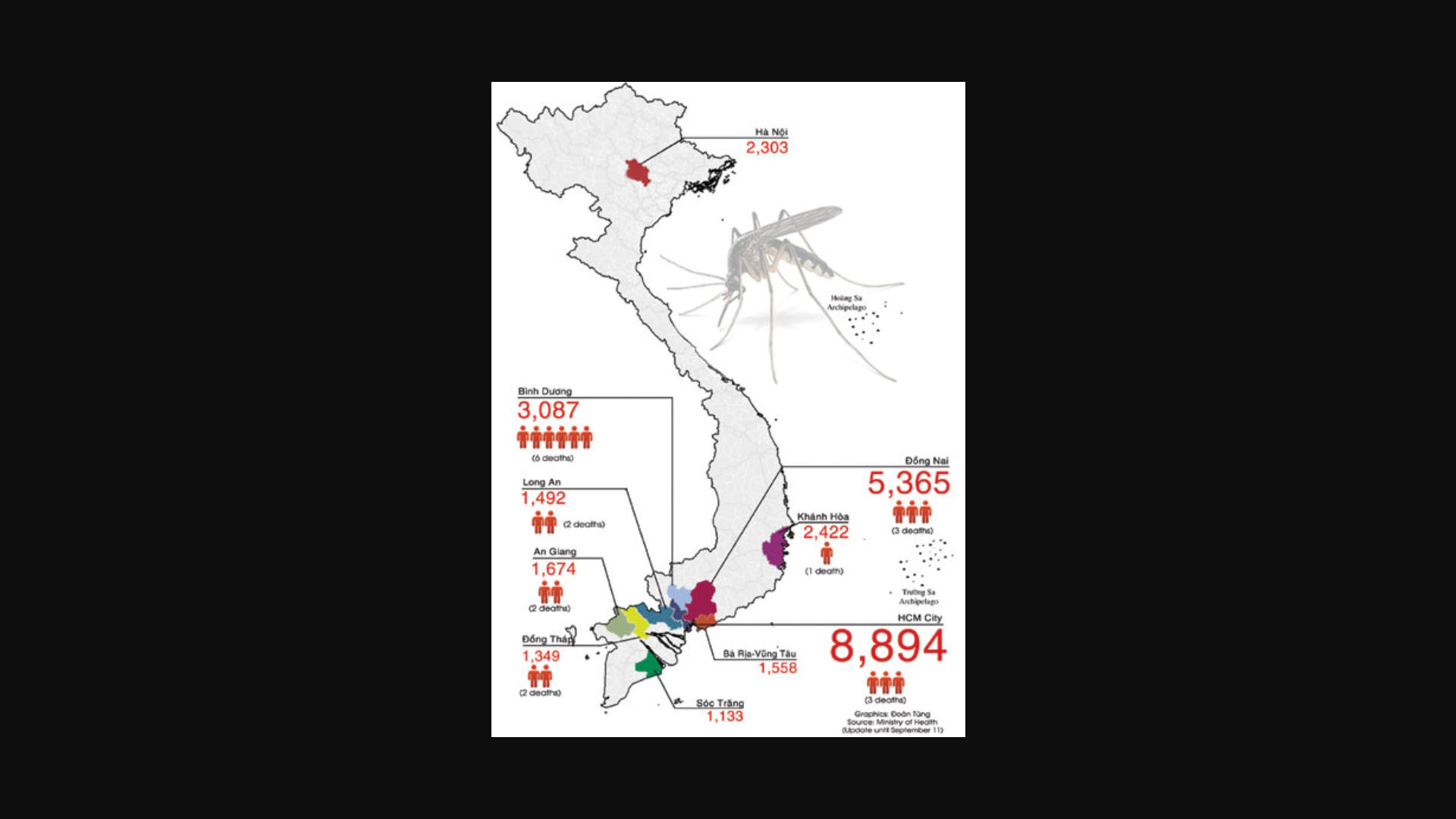
Since September 27, 2024, when the Rwanda Ministry of Health confirmed the country's first outbreak of MVD, 61 cases and 14 related deaths have been reported.
As of October 11, 2024, no approved MVD vaccines exist, but experimental vaccines are being tested in Rwanda.
Orlance, Inc. today announced it was awarded the National Institutions of Health (NIH) Fast Track Small Business Innovation Research (SBIR) grant to develop an Enhanced Seasonal Influenza Vaccine.
This vaccine is intended to provide better protection against disease even in years when predicted vs. actual circulating strains are highly mismatched.
The award includes $300,000 for Phase 1, with the total funding for the Phase 1 and 2 combined program amounting to $3.3 million.
This Fast Track SBIR grant will enable Orlance to leverage its innovative MACH-1 powdered vaccine and immunotherapy platform to address seasonally changing and highly conserved influenza immunogens in ways that are impossible with other platforms.
Specifically, this program builds upon Orlance's universal influenza vaccine, which targets conserved antigens consistent across multiple virus lineages and adds seasonally changing influenza antigens to maximize protection.
The MACH-1 platform is a high-performance microparticle 'gene gun' technology that efficiently and uniquely delivers DNA or RNA vaccine-coated microparticles directly into cells in the uppermost layer of the skin.
MACH-1 delivery harnesses this environment and the natural machinery of its immune cells to deliver DNA and RNA vaccines encoding proteins that trigger potent immunity, including antibodies to block an infection and T cells that can eliminate infected cells.
Unlike currently licensed mRNA vaccines, MACH-1-delivered vaccines are stable at room temperature, painless, and needle-free. Orlance MACH-1 vaccines also trigger protective immunity levels with the smallest doses achieved within the field.
"NIH's continued funding support of the MACH-1 platform, including this enhanced seasonal influenza vaccine, reinforces the potential impact and significant step forward MACH-1 can bring to vaccine technology," adds Kristyn Aalto, Orlance CEO, in a press release on October 10, 2024.
This award brings Orlance's SBIR funding to $16.8M for next-generation generation DNA and RNA vaccines and therapeutics.

A report published today by the World Health Organization (WHO) finds that vaccines against 24 pathogens could reduce the number of antibiotics needed by 22% or 2.5 billion defined daily doses globally every year.
The WHO says vaccinated people have fewer infections.
Announced on October 10, 2024, this technical report supports the worldwide efforts to address antimicrobial resistance (AMR). Each year, nearly 5 million deaths are associated with AMR globally.
AMR occurs when bacteria, viruses, fungi, and parasites no longer respond to antimicrobial medicines. This makes people sicker and increases the risk of illness, death, and the spread of difficult-to-treat infections.
Vaccines are an essential part of the response to reduce AMR. They prevent infections, reduce the use and overuse of antimicrobials, and slow the emergence and spread of drug-resistant pathogens.
While some vaccines are already available but underused, other innovative vaccines must be developed as soon as possible, wrote the WHO.
Dr Tedros Adhanom Ghebreyesus, WHO Director-General, commented in a press release, “Prevention is better than cure, and increasing access to existing vaccines and developing new ones for critical diseases, like tuberculosis, is critical to saving lives and turning the tide on AMR.”
The new report expands on a WHO study published in BMJ Global Health in 2023.

The U.S. FDA's Center for Biologics Evaluation and Research is conducting the 187th meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) today.
This committee of vaccine experts will discuss the strain selection for Influenza Virus Vaccines for the 2025 Southern Hemisphere flu season on October 10, 2024. Each year, the VRBPAC discusses how the influenza virus is evolving and if it impacts exciting flu shot efficacy.
On October 3, 2024, the U.S. CDC reported that five Southern Hemisphere countries reported flu shots were about 35% effective during the last flu season.
Previously, on September 27, 2024, the World Health Organization announced its recommendations for the viral composition of influenza vaccines for the 2025 influenza season in the Southern Hemisphere.
Another presentation on the agenda is the 'Highly Pathogenic Avian Influenza A(H5Nx) Virus Surveillance and Characterization' in the U.S. and globally, and review recommendations for candidate vaccine virus development.
Over the past few months, the U.S. government has substantially invested in developing preventive vaccines for a potential avian influenza pandemic.
Instructions on listening to this VRBPAC meeting are found at this FDA link.

Thousands of healthcare professionals, advocates, and public policymakers from over 170 countries are participating in the annual Global TB Summit as the world confronts the resurgence of tuberculosis as the second deadliest infectious disease.
After a decline of about 2% per year between 2020 and 2022, the global tuberculosis incident rate rose by 3.9% in 2022. An estimated 10.6 million people worldwide fell ill with tuberculosis in 2022, leading to 1.3 million deaths.
“Tuberculosis remains a significant global threat, even though it is both preventable and curable. At the Global TB Summit 2024, we focus on the most pressing challenges. Our mission is clear: to harness innovation and foster global collaboration in our fight against this deadly disease. Only by coming together and leveraging our collective expertise can we seek to have a TB-free world,” said Glen Hansen, Chief Medical Officer at QIAGEN, in a press release on October 7, 2024.
After 27 years of declining tuberculosis cases, in 2023, the 50 U.S. states and the District of Columbia provisionally reported 9,615 TB cases, representing an increase of 16% compared with 2022.
Many of the cases were confirmed in children living with adults infected with TB.
The U.S. CDC stated in March 2024, 'Continued progress toward TB elimination will require strong public health systems capable of maintaining essential disease prevention and control activities.'
One of the prevention options is vaccination since TB is a vaccine-preventable disease.
Globally, there are over ten tuberculosis vaccines available in 2024.
In the U.S., the BCG vaccine is not commercially available or integrated into the CDC's vaccination schedule for children.
Novavax, Inc. today announced that the European Commission granted Marketing Authorization for Novavax's updated 2024-2025 Nuvaxovid™ COVID-19 Vaccine for use in individuals aged 12 and older to prevent COVID-19 in the European Union.
As of October 9, 2024, Novavax's vaccine is in line with guidance from the U.S. Food and Drug Administration and the World Health Organization to target the SARS-CoV-2 virus JN.1 lineage during the fall of 2024.
In the U.S., Novavax is the only protein-based vaccine available in pharmacies such as CVS Pharmacy, Giant, Publix, and Rite Aid.

The U.S. Centers for Disease Control and Prevention (CDC) announced yesterday that international travelers should reconsider nonessential travel to the Republic of Rwanda, which is experiencing an outbreak of Marburg virus disease.
As of October 8, 2024, Rwanda's Health Ministry posted on X that there have been 58 MVD cases and 13 related fatalities over the past month.
Rwanda has been screening departing travelers to reduce the global outbreak risk.
Starting the week of October 14, 2024, the U.S. will begin public health entry screening of travelers entering the U.S. who have been in Rwanda in the past 21 days. This screening will be conducted at Chicago's O'Hare International Airport, New York City's John F. Kennedy International Airport, and the Washington, DC Dulles International Airport.
"This screening aims to reduce the risk of importation of Marburg cases into the United States and the spread within U.S. communities," announced HHS in a statement. When passengers arrive at one of these airports, they will meet with CDC staff for an assessment.
The CDC says Marburg is a viral hemorrhagic fever, with symptoms including fever, chills, headache, muscle aches, rash, chest pain, sore throat, nausea, vomiting, diarrhea, or unexplained bleeding or bruising.
There are no approved vaccines for Marburg, but various clinical trials are ongoing. Recently, 700 experimental Marburg vaccines were sent to Rwanda for testing.