Search API
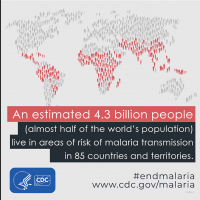
In continued collaboration with the Republic of Rwanda, the Sabin Vaccine Institute announced it dispatched approximately 1,000 additional investigational vaccine doses for a randomized clinical trial arm within the ongoing open-label study targeting Marburg virus disease, which causes deadly viral hemorrhagic fever.
On October 31, 2024, Sabin confirmed that over 1,700 vaccines had already been delivered to Rwanda since September 27. The initial part of the trial focused mainly on health workers, who suffered the most casualties in this outbreak.
Rwanda has confirmed 66 Marburg cases and 15 related deaths in one of the most significant recorded outbreaks of this disease, which was first detected in Germany in 1967.
Marburg is spread by contact with objects, blood, or body fluids of a person infected with or who has died from Marburg.
Designed to prevent illness before exposure to the virus, Sabin’s Marburg vaccine based on the cAd3 platform has not yet been proven to have clinical benefit for vaccine recipients. The candidate is currently in Phase 2 trials in Uganda and Kenya ; no safety concerns have been reported. In Phase 1 trials, safety and immunogenicity were shown in humans.
Sabin is also a key partner in MARVAC, a WHO-coordinated effort promoting global collaboration in Marburg vaccine development.
As of November 1, 2024, the U.S. CDC says, 'Reconsider nonessential travel to the Republic of Rwanda, which is experiencing an outbreak of Marburg virus disease.'
As the world confronts dengue fever outbreaks in over 100 countries, many are launching vaccination campaigns to reduce the impact of this mosquito-transmitted disease.
And a Japan-based company is benefiting from increased vaccine sales.
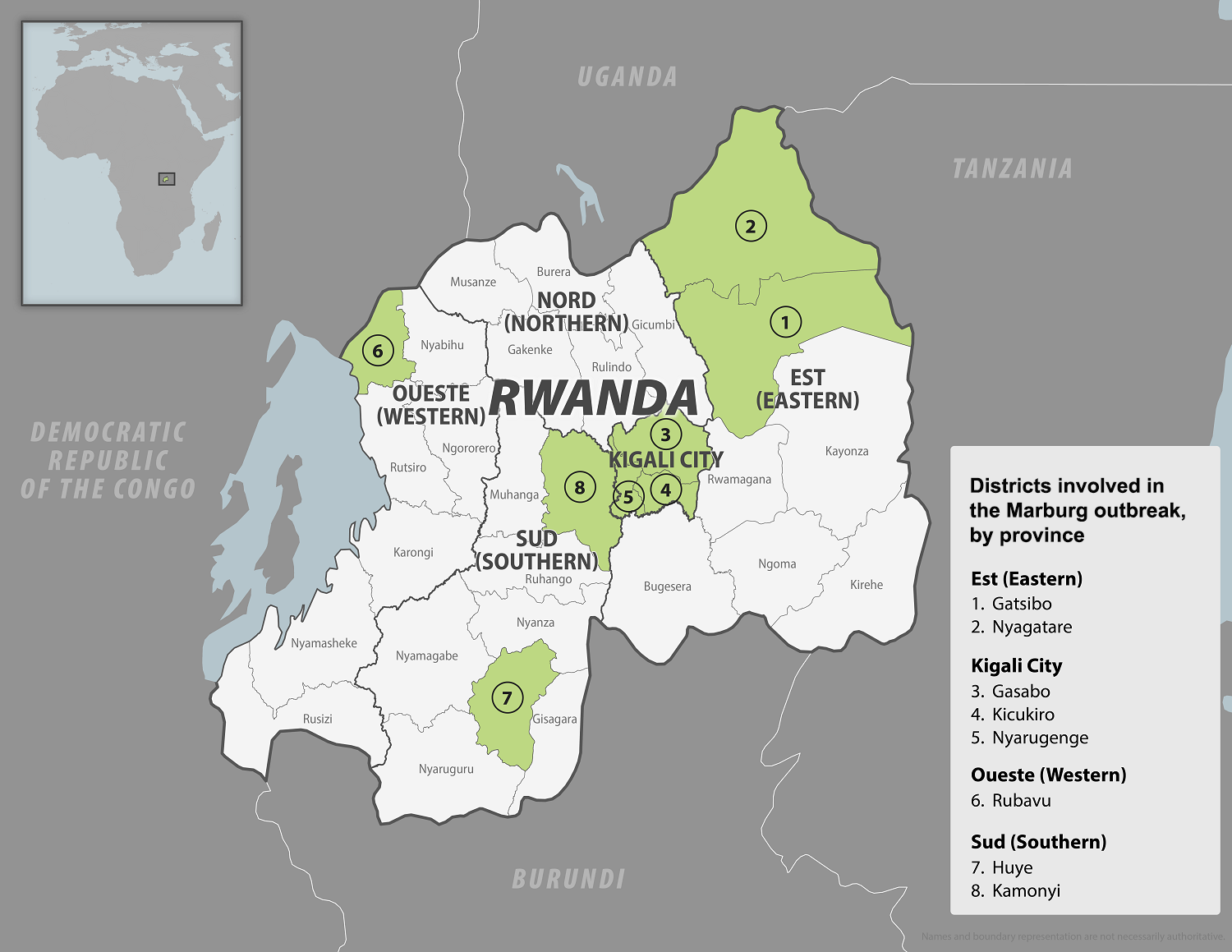
Takeda Pharmaceutical Company Limited today announced that its dengue vaccine QDENGA sales increased 863% in the FY2024 H1, reaching JPY 19.9 billion (~$124 million).
On October 31, 2024, Takeda's quarterly presentation revealed on slide #29, the following insights:
QDENGA is now available in 25 countries, including 18 European countries. Increasing breadth and depth in these markets and further expansion into Malaysia, Israel, and Vietnam in 2024.
Previously, the World Health Organization added QDENGA to its List of Prequalified Vaccines, and the Gavi Board recently approved support for a dengue vaccine program.
In the United States, where QDENGA is unavailable, the Centers for Disease Control and Prevention (CDC) has reported 6,819 travel-related and locally acquired dengue cases in 50 jurisdictions this year, led by California, Florida, New Jersey, New York, and Puerto Rico.
This CDC data indicates an 11% increase compared to 2023, when 6,164 dengue cases were reported.
The U.S. CDC published a new Morbidity and Mortality Weekly Report (73(6);1–18) today that confirmed from the 2010–11 through 2022–23 influenza seasons, older adults (aged ≥65 years) consistently had the highest laboratory-confirmed influenza-associated hospitalization rates than other age groups.
The CDC wrote on October 31, 2024, that studies have demonstrated that vaccinated, hospitalized adult patients have a 26%–59% reduced risk of being admitted to the ICU and a 31% reduced risk of death compared with unvaccinated patients.
This data revealed many seniors were unprotected against influenza infection.
Adults aged ≥65 who were hospitalized with influenza consistently had the highest proportion of current-season influenza vaccine receipt (range = 40.9% during 2022–23 to 60.7% during 2018–19).
According to the CDC's Advisory Committee on Immunization Practices meeting last week, all persons should receive influenza vaccinations. Still, it is essential for those with underlying medical conditions to be vaccinated because of the elevated risk of complications from an influenza virus infection.
As of the end of October, about 100 million flu shots had been distributed to healthcare providers in the U.S. These vaccines are generally available at clinics and pharmacies.

A stay in a hospital might resolve one health challenge. Still, it may introduce another: an intractable infection with Clostridioides difficile (C difficile), wrote an Editors Summary published in the journal Science in October 2024.
This bacteria can thrive and produce toxins if antibiotics hinder the normal gut bacteria.
Alameh et al. disclosed they have been developing a multivalent mRNA–lipid nanoparticle vaccine to protect vulnerable individuals against toxigenic C. difficile. The University of Pennsylvania researchers designed the vaccine candidate to target an enzyme found in diverse strains of this bacterium that processes several surface factors required for gut colonization and virulence.
They concluded, 'Our studies demonstrate mRNA-LNP vaccine technology as a promising platform for developing novel C. difficile therapeutics with potential for limiting acute disease and promoting bacterial decolonization.'

The U.K. Health Security Agency (UKHSA) announced today that it had detected its first Clade Ib mpox human case in London, England.
As of October 30, 2024, the infected individual has been transferred to the Royal Free Hospital High Consequence Infectious Diseases unit.
This person had recently traveled to countries in Africa that are reporting Clade Ib mpox outbreaks.
The UKHSA and NHS stated that the risk to the U.K. population remains low and will not disclose further details about this case. Any contacts will be offered testing and vaccination as needed and advised on any necessary further care if they have symptoms or test positive.
This is the first detection of this Clade of mpox in the U.K. It differs from mpox Clade II, which has circulated at low levels in the U.K. since 2022.
U.K. Health and Social Care Secretary Wes Streeting commented in a press release, "The overall risk to the U.K. population currently remains low... (We) are securing vaccines and equipping healthcare professionals with the guidance and tools to respond to cases safely."
According to a recent World Health Organization analysis, Clade 1 viruses have been detected in Central Africa for decades.
In the United States, the CDC reported on October 19, 2024, that 2,230 Clade II cases were confirmed in 2024, compared with 1,096 reported in 2023.
In the U.S., Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) two-dose mpox/smallpox vaccine is commercially available at clinics and pharmacies. Vaccine efficacy data against Clade Ib is pending.

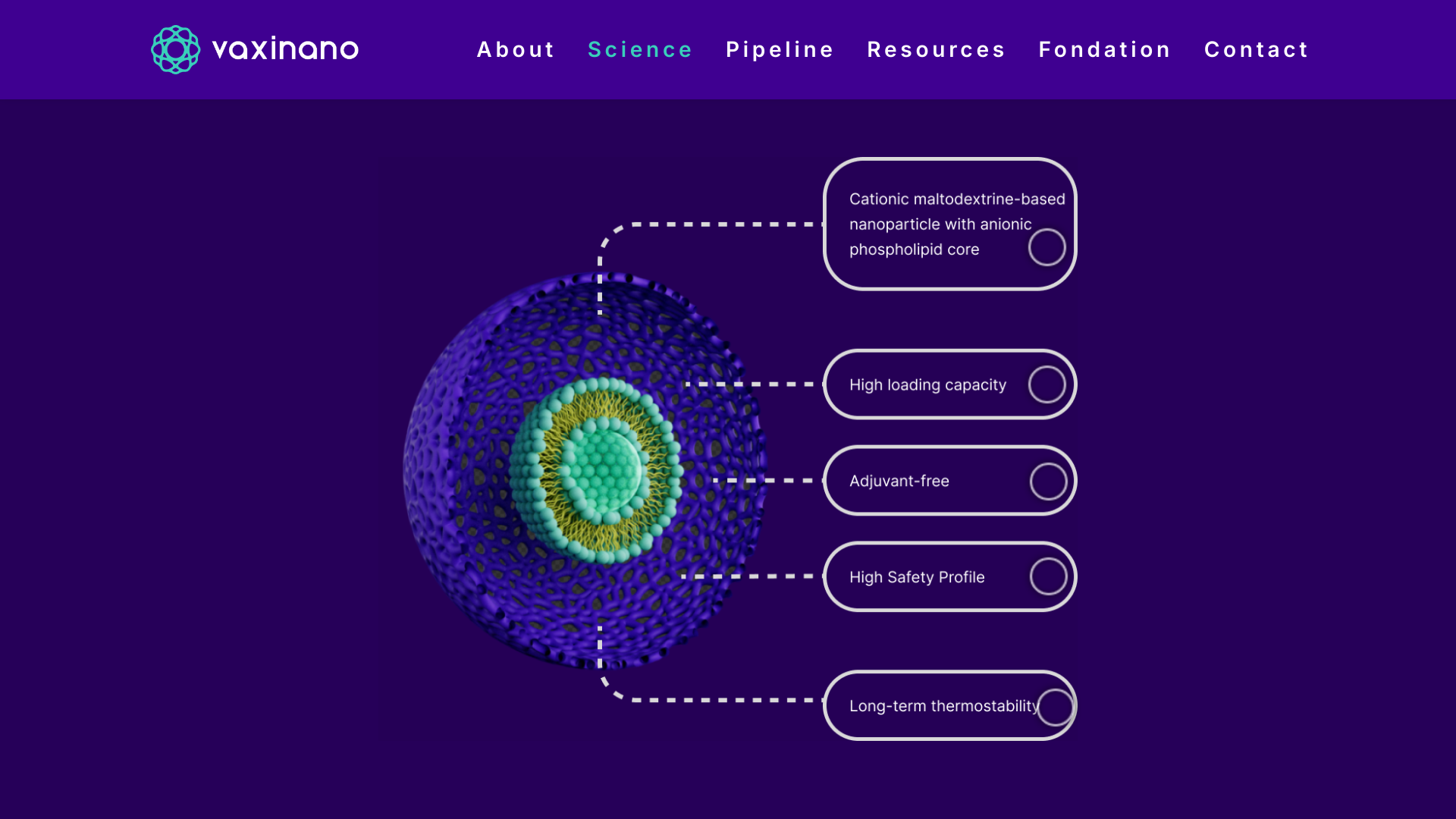
Vaxinano, a biotech company specializing in developing prophylactic and therapeutic nasal vaccines, has successfully raised €6 million in a funding round.
Thanks to its Stellar-NP nanoparticle technology, Vaxinano is developing a new generation of highly stable, adjuvant-free nasal vaccines designed to deliver effective and long-lasting responses to the most challenging pathogens, including parasites, viruses, and bacteria.
The platform has demonstrated preventive and therapeutic efficacy across multiple species and various infectious diseases, such as leishmaniasis, toxoplasmosis, and colibacillosis.
Didier Betbeder, founder and CSO of Vaxinano, stated in a press release on October 29, 2024, "The Stellar-NP technology has the potential to revolutionize the vaccine landscape by providing more effective and cost-efficient solutions in a context of emerging pandemics."
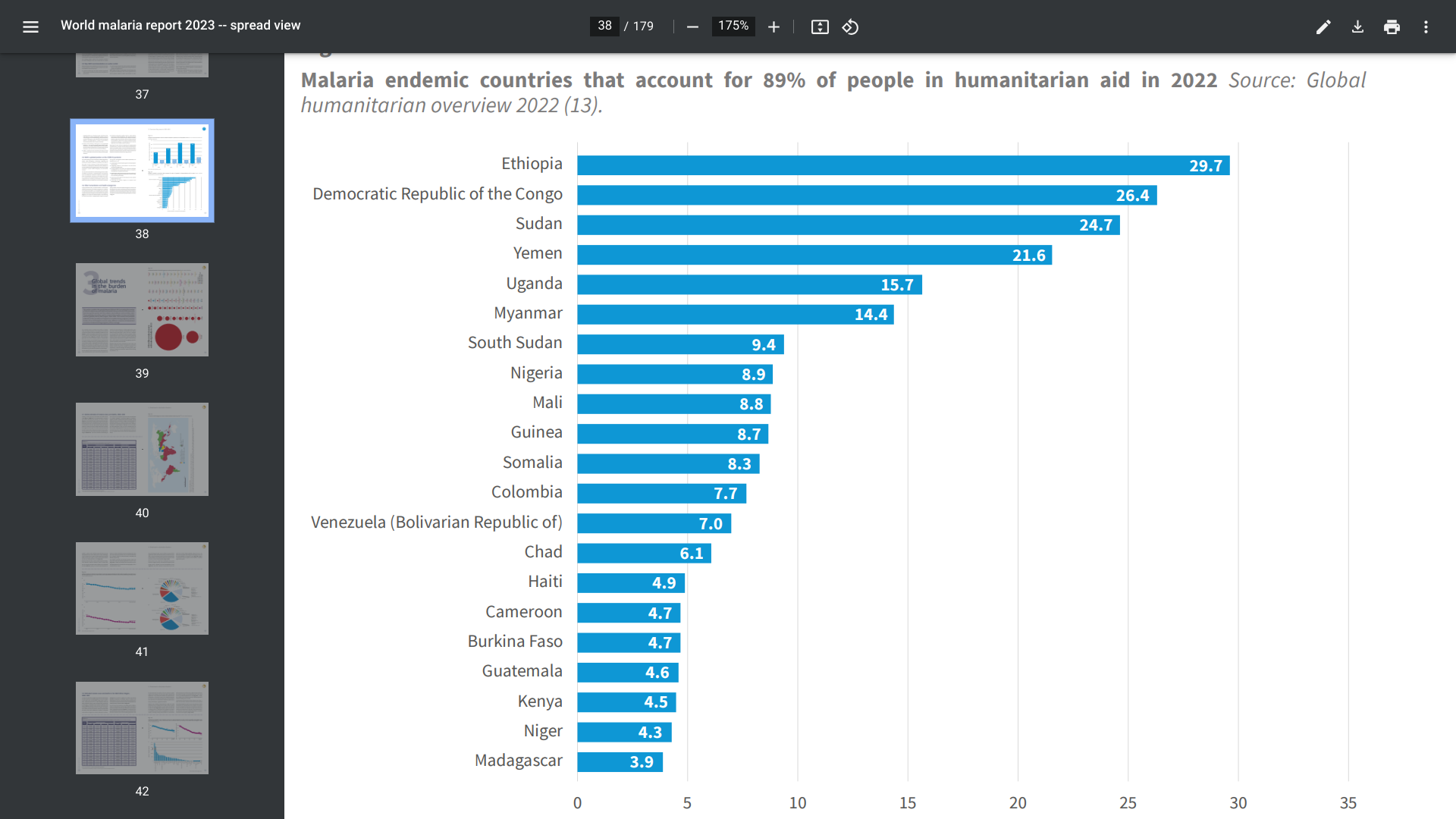
Malaria vaccines have been in clinical development since the 1960s, with substantial progress over the past few years. In October 2021, the first of two malaria vaccines was approved to prevent Plasmodium falciparum malaria in children living in regions with moderate to high transmission.
However, recent studies have found maternal antibodies passed to infants can interfere with the response to the malaria vaccine.
The lower antibody titers in infants were attributed to either co-administration with routine vaccines included in the WHO Expanded Programme on Immunization, maternal anti-CSP antibodies, immune status regarding previous exposure, the infant's immature immune system, or a combination of these factors.
Published on October 23, 2024, this observational study conducted in six African countries, researchers concluded that interference between passive immunity and vaccine response is clinically significant and might affect the implementation of next-generation CSP-based vaccines for young infants and mothers and passive immunization with human monoclonal antibodies.
To validate this conclusion, additional clinical studies are being conducted.
As of late October 2024, malaria vaccines are offered in Africa, not the United States.