Search API
With over 1.1. billion doses already delivered, the Global Health Technologies Coalition (GHTC) recently honored the novel oral polio vaccine type 2 (nOPV2) development consortium with its 2024 Innovating for Impact Award.
The GHTC awards, issued annually in December, recognize multisectoral partnerships and policymakers helping to transform breakthrough scientific research into lifesaving drugs, diagnostics, vaccines, and other health tools for unmet global health challenges.
Dr. Ananda Bandyopadhyay, Deputy Director of Technology, Research, and Analytics, Polio Team, Bill & Melinda Gates Foundation, commented in an announcement, "The fight against polio has always been a story of partnerships…So many countries, partners, and people came together to develop this vaccine. This nOPV2 journey is an example of pushing the boundaries of innovation and doing it as a global team."
nOPV2 is derived from the live, infectious virus and has been 'triple-locked' using genetic engineering to prevent it from producing mutations and causing paralysis. As a result, nOPV2 is reported to be more genetically stable than previous oral polio vaccines.
The WHO's SAGE recently recommended that, where feasible, the concomitant use of Inactivated polio vaccine (IPV) and nOPV2 be used for initial poliovirus outbreak response vaccination campaigns.
The IPV has been offered in the U.S. since 2000, while the nOPV2 has been provided in Africa in 2024.
Polio is a very contagious infectious disease that can lead to permanent paralysis. Worldwide polio cases have dropped by 99% since 1988 thanks to global vaccination efforts.

A shipment of 11,200 vaccine doses, donated by the United States of America, has been shipped to Abuja, Nigeria, to curtail the ongoing clade 1 mpox outbreak.
This shipment, announced on December 20, 2024, follows agreements signed in November by Gavi, the Vaccine Alliance, to facilitate the donation of 305,000 mpox vaccine doses to support the global and continental response.
In September 2024, the U.S. announced its intention to donate up to 1 million JYNNEOS® (MVA-BN®) doses to support the mpox emergency.
Assistant Secretary for Preparedness and Response (ASPR) Dawn O'Connell commented in a press release, "Viruses don't respect borders, and both international and domestic mpox coordination remains a top priority for ASPR."
Two types of the virus cause mpox, clade I and clade II. Both types spread the same way and can be prevented using innovative vaccines.
The first case of clade I mpox in the U.S. was detected in November 2024 following the patient's travel to an affected area. No additional cases were reported.
The initial case of clade IIb mpox in the U.S. (Boston) was in May 2022. According to the U.S. CDC, the ongoing global outbreak of clade II mpox has caused more than 100,000 cases in 122 countries and continues in the U.S.
Bavarian Nordic's JYNNEOS® two-dose vaccine is based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara, and is commercially available in the U.S.
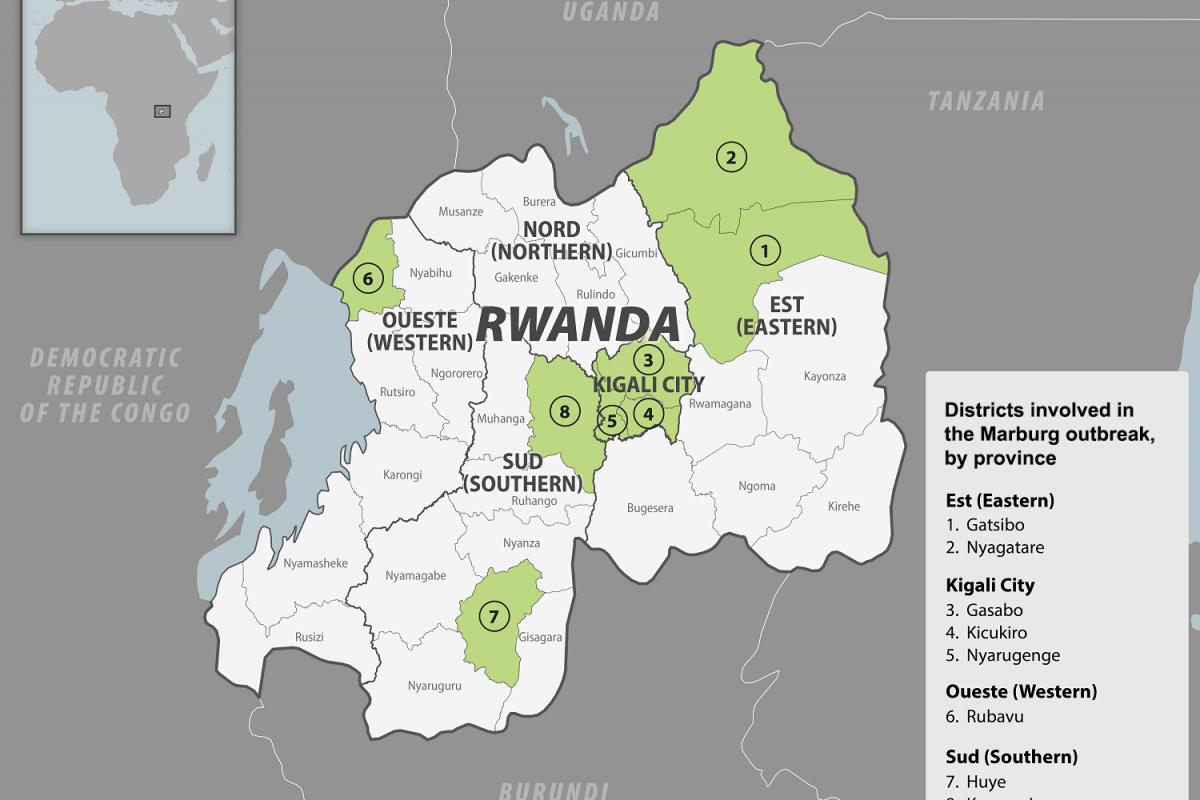
After 42 days without detecting a new case, the World Health Organization (WHO) announced that the Marburg Virus Disease (MVD) outbreak in the Republic of Rwanda had ended.
The outbreak, confirmed in late September 2024, was the first Marburg Virus Disease outbreak Rwanda has experienced. A total of 66 confirmed cases and 15 deaths (23%) were recorded. Almost 80% of the cases were among infected health workers while providing clinical care to their colleagues and other patients.
"The robust response by Rwanda shows how committed leadership, concerted efforts by partners, and a strong health system are crucial in addressing public health emergencies, saving and protecting lives, as well as safeguarding the health of individuals and communities," said Dr. Brian Chirombo, WHO Representative in Rwanda, in a media release issued on On December 20, 2024.
The virus which causes Marburg is in the same family as the virus that causes Ebola Virus Disease. Marburg virus is transmitted to people from fruit bats and spreads among humans through direct contact with the bodily fluids of infected people, surfaces, and materials.
In October 2024, the Sabin Vaccine Institute announced it dispatched investigational vaccine doses for a randomized clinical trial targeting Rwanda's outbreak.
As of December 2024, there are no approved Marburg virus vaccines.
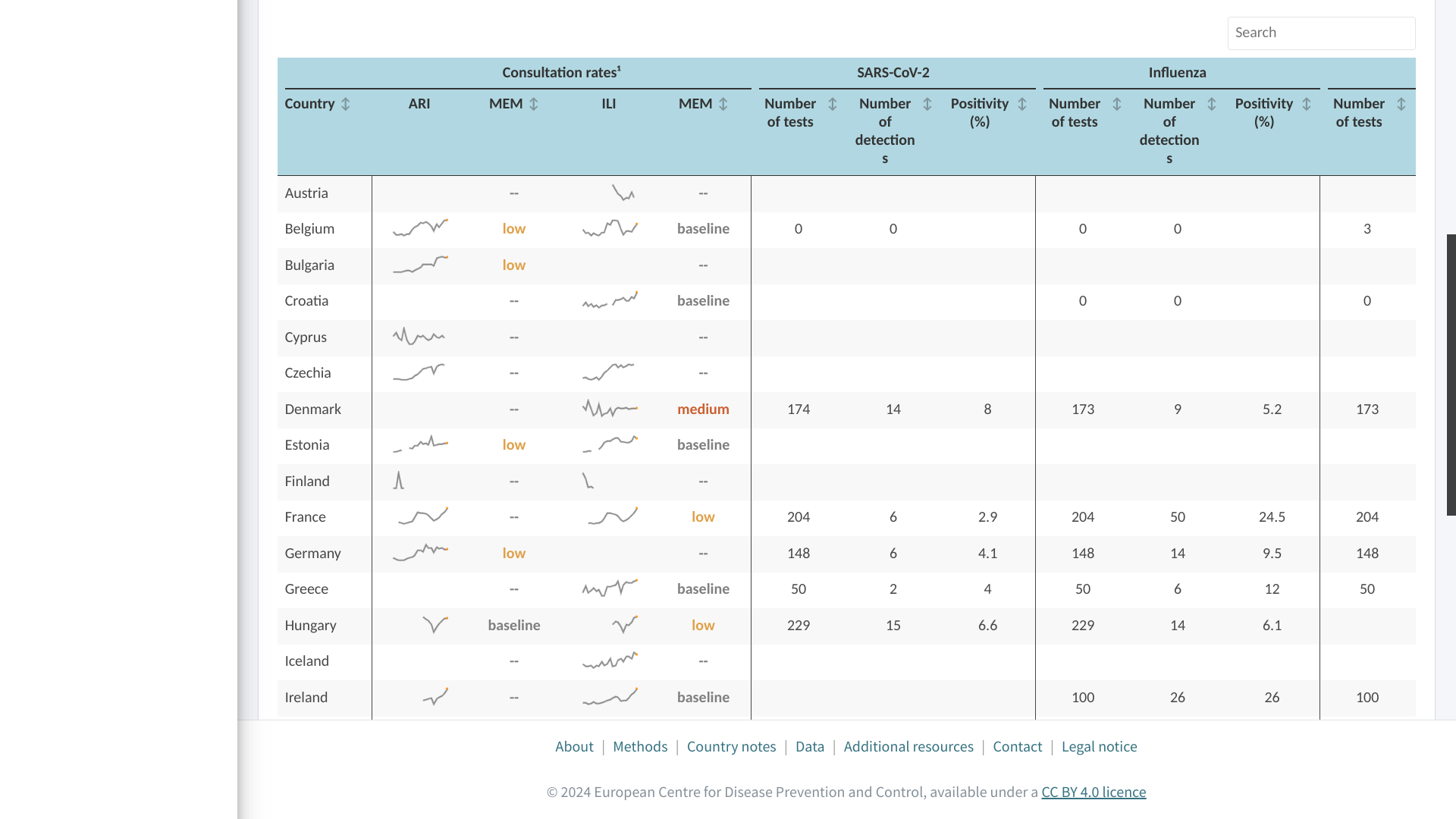
As the end-of-year festive season is traditionally associated with social gatherings and traveling, the Centre for Disease Prevention and Control (ECDC) says these endeavors pose additional risks for intensified respiratory virus transmission.
On December 18, 2024, almost all countries reporting data to ECDC (week 49, 2024) observed sharp increases in indicators of influenza and respiratory syncytial virus (RSV) activity.
The EU/EEA 10% primary care test positivity threshold signaling the start of the influenza season has been reached.
Furthermore, the impact of influenza may be worse if an A(H3N2) subclade that is less well matched with the northern hemisphere vaccine(s) dominates.
Co-circulation of influenza viruses and RSV could substantially impact healthcare services. Hospital admissions could occur in all age groups, with very young children (due to RSV) and older adults particularly affected.
The ECDC joins the U.S. CDC in encouraging all eligible people to protect themselves with approved vaccines for the 2024-2025 respiratory season.
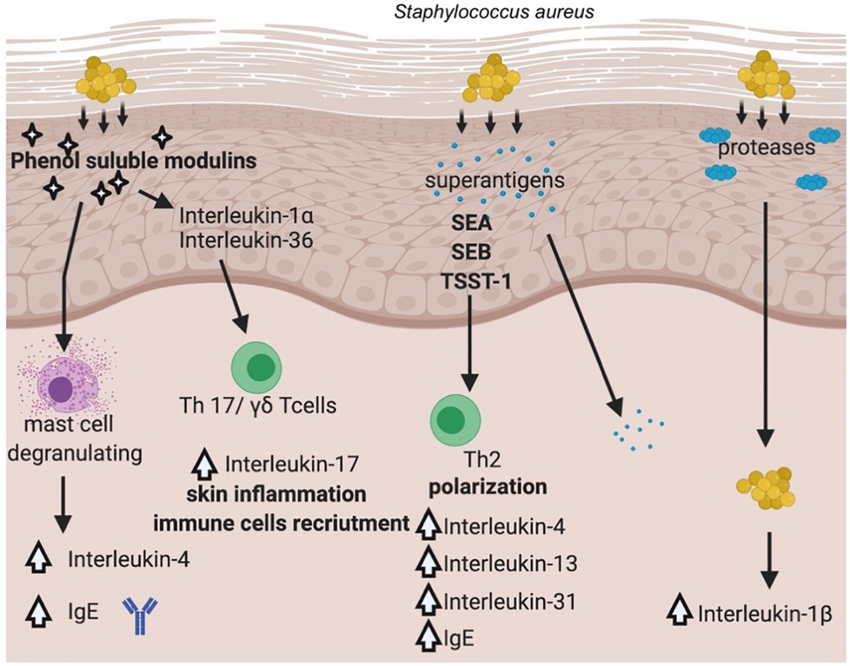
LimmaTech Biologics AG announced today that the U.S. Food and Drug Administration had granted Fast Track designation to the company's multivalent toxoid vaccine candidate, LBT-SA7.
This innovative vaccine is designed to prevent skin and soft tissue infections (SSTIs) caused by the bacterial pathogen Staphylococcus aureus (S. aureus).
It is estimated that more than 1 million deaths are attributed to S. aureus each year. Notably, 90% of all community-acquired S. aureus infections are SSTIs.
S. aureus has been designated as a "high priority" pathogen by the World Health Organization, highlighting the urgency for innovative vaccine approaches and effective treatment strategies.
Dr. Franz-Werner Haas, CEO of LimmaTech, commented in a press release on December 19, 2024, "Staphylococcus aureus infections are a major cause of global mortality and morbidity, with traditional antibiotic treatments becoming increasingly ineffective due to rising antibiotic resistance."
LBT-SA7 will be tested in a Phase 1 study at a clinical trial center in the U.S., including 130 adult participants, with initial results anticipated in the second half of 2025.
The U.K. Travel Health Pro confirmed today that all travelers to areas where the Zika virus is known to occur are at risk of infection, although determining the actual level of risk is complex.
For example, travelers who spend even short-term vacations in endemic areas may be exposed to the mosquito-transmitted virus.
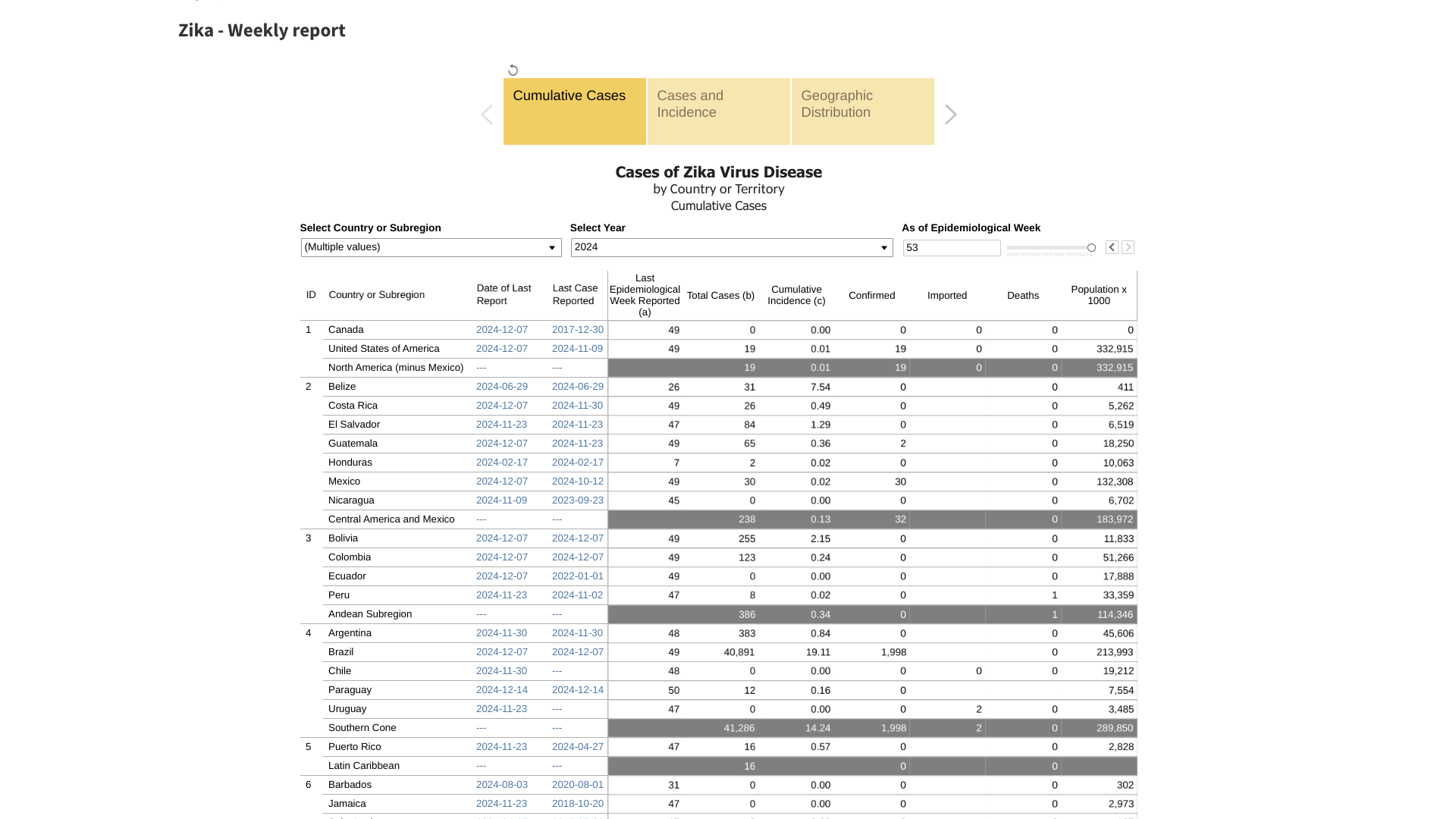
On December 18, 2024, the U.K. highlighted Zika cases in the Region of the Americas, specifically in the Federative Republic of Brazil.
So far this year, over 40,891 cases have been reported. Last year, the PAHO had 35,962 Zika cases.
This PAHO data indicates a 14% increase in Zika cases over 2023.
Seperately, the U.S. CDC reported 19 non-congenital Zika cases in U.S. residents (1 imported case in Texas) in 2024. In 2023, the CDC reported five non-congenital cases in U.S. residents and 27 in U.S. territories.
In the U.S. Territory of Puerto Rico, the Department of Health says that Zika-spreading mosquitoes are found throughout the island.
The CDC says that if you are pregnant, you should avoid traveling to Zika outbreak destinations. If travel is unavoidable, you should strictly follow Zika prevention recommendations.
From a disease prevention perspective, Zika vaccine candidates continue conducting clinical trials, but none have been approved for use in the U.S.
Several cases of East African sleeping sickness have been reported among travelers returning from areas in Zambia and Zimbabwe, which often conduct safaris for visitors.
According to the WHO, sleeping sickness, also called African trypanosomiasis, is caused by a parasite transmitted by an infected tsetse fly, which is found only in sub-Saharan Africa.
There are two types of sleeping sickness, East African and West African. East African sleeping sickness progresses more quickly, within one to several weeks of exposure.
The U.S. Centers for Disease Control and Prevention (CDC) announced on December 18, 2024, that it had issued a Level 1 - Practice Usual Precautions, Travel Health Advisory regarding this unusual, potentially fatal situation.
Expidiated diagnosis and treatment can be lifesaving.
According to the WHO, anti-trypanosomals are donated and distributed free to endemic countries.
The CDC wrote that people should seek medical care immediately if they develop headache, fever, fatigue, skin rash, muscle aches, or a red sore, called a chancre, during or after travel to safari regions of Zambia or Zimbabwe, and they think a tsetse fly may have bitten you.
As of December 2024, no vaccines are authorized to prevent either type of sleeping sickness.

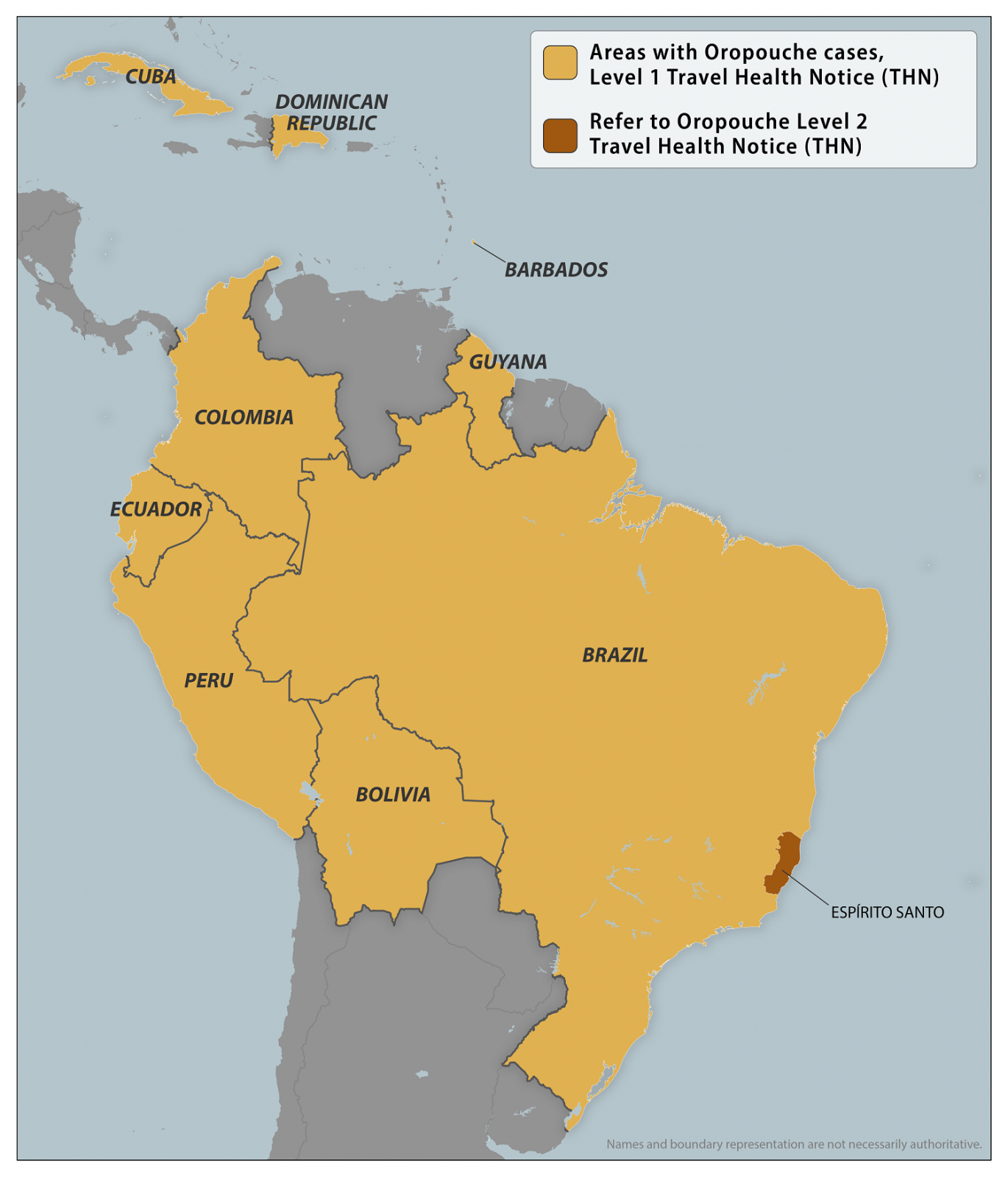
The U.S. Centers for Disease Control and Prevention (CDC) today announced it updated its Level 1 - Practice Usual Precautions, Travel Health Advirus for Oropouche virus outbreaks in numerous countries in the Region of the Americas.
On December 18, 2024, nine countries have reported Oropouche cases, including related fatalities.
A Level 2 Notice was previously issued for Oropouche in Espírito Santo, Brazil.
Oropouche virus is spread primarily through the bites of infected midges and mosquitoes. Illness can occur in people of any age and is often mistaken for dengue.
The CDC wrote that travelers to affected areas should avoid bug bites during travel to protect themselves from infection. They should also prevent bug bites for three weeks after travel to avoid possibly spreading the virus to others in the U.S.
Most people recover without long-term effects.
Furthermore, if travelers are pregnant, they should discuss travel plans, reasons for travel, steps to prevent bug bites, and potential risks with their healthcare provider. While it is unknown if Oropouche can be spread by sex, initial reports indicate infants have also been infected with this virus.
The CDC is working with PAHO and other international partners to learn more about the potential risks of Oropouche during pregnancy.
As of late December, no vaccines or targeted therapies are available for Oropouche disease.