Search API
The U.S. Centers for Disease Control and Prevention (CDC) today confirmed an ongoing outbreak of clade I mpox in Central and Eastern Africa. Previous data indicates that about 15 million people visit central Africa each year.
As of April 1, 2025, the CDC updated its Level 2 Travel Health Advisory saying, 'There is ongoing person-to-person transmission of mpox in Burundi, Central African Republic, Democratic Republic of the Congo (DRC), Kenya, the Republic of the Congo, Rwanda, Tanzania, Uganda, and Zambia.'
Person-to-person transmission has occurred through various means during this outbreak.
There are two types of Monkeypox virus. Historically, clade I has been associated with a higher percentage of people with mpox developing severe illness or dying, compared to clade II. The global outbreak of clade II began in May 2022.
The CDC writes, 'Mpox vaccination is recommended for people who anticipate the following sexual activities during travel to countries with ongoing person-to-person transmission of clade I mpox.'
In the United States, the Bavarian Nordic JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) two-dose vaccine is commercially offered at various travel clinics and pharmacies in April 2025.
Those eligible for mpox vaccination should get two doses of JYNNEOS at least 28 days apart, before visiting an mpox outbreak area.

As the global outbreak of the Chikungunya virus continues to expand in India, Brazil, and La Réunion, European adolescents can now receive an effective vaccine that protects them from this mosquito-transmitted disease.
On April 1, 2025, Valneva SE announced that the European Commission (EC) has granted marketing authorization in Europe for Valneva’s single-dose vaccine, IXCHIQ®, for the prevention of disease caused by the chikungunya virus in individuals 12 years of age and older.
With this approval, IXCHIQ® will become available for adolescents in the EU, Norway, Liechtenstein, and Iceland.
In addition to the EC's approval in adolescents and adults in the EU, it has been approved in the United States, Canada, and the United Kingdom to prevent diseases caused by the chikungunya virus in individuals 18 and older. Similar label extension applications for adolescents were also submitted in the U.S., Canada, and the U.K.
Dr. Richard Hatchett, Chief Executive Officer of the Coalition for Epidemic Preparedness Innovations, commented in a media release, “Cases of chikungunya are increasing around the world, making populations of all ages vulnerable to the disease’s long-term debilitating effects, such as prolonged joint pain and inflammation."
"EC’s marketing authorization for use of IXCHIQ® in adolescents in the EU is an important stepping stone that could help accelerate the approval of the vaccine in this age group in other regions, including areas where the disease is endemic.”
In the United States, IXCHIQ is commercially available at most travel clinics and pharmacies and is recommended by the U.S. CDC for people visiting Chikungunya endemic areas in 2025.

With clade I mpox outbreaks occurring in hard-to-reach areas in Central and Eastern Africa, a new version of an effective vaccine has been approved.
Bavarian Nordic A/S announced that the U.S. Food and Drug Administration (FDA) has approved the freeze-dried formulation of the JYNNEOS® vaccine to prevent smallpox and mpox disease in adults 18 and older.
Announced on March 31, 2025, this FDA approval will enable additional flexibility for stockpiling against a smallpox event or mpox outbreak.
The Company recently informed the African CDC that it can manufacture about ten million vaccine doses in addition to current orders by the end of 2025.
The current liquid-frozen formulation of JYNNEOS, approved by the FDA in September 2019, has specific cold-chain requirements, while the freeze-dried formulation provides advantages in terms of transportation, storage conditions, and shelf life, all of which are essential factors for long-term stockpiling.
“Today’s FDA approval represents a significant milestone in our development of this next generation of JYNNEOS and in our collaborative efforts with the U.S. government to strengthen public health security,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release.
“As a long-term supplier of JYNNEOS to the U.S. biological preparedness, we are committed to supporting the government’s efforts to protect its citizens against current and future public health threats.”
To alert international travelers of this continued health risk, the U.S. CDC maintains Level 2 Travel Health Advisories.
JYNNEOS, a two-dose vaccine based on a live, attenuated vaccinia virus, is commercially available in pharmacies and clinics in the United States.
Internationally, JYNNEOS is known as MVA-BN®, IMVAMUNE®, and IMVANEX®.

Following the launch of the Global Polio Eradication Initiative in 1988, the number of paralytic poliomyelitis cases was reduced by about 99% globally, with wild-type PV1 remaining endemic in only Afghanistan and Pakistan.
However, the World Health Organization recently reconfirmed that the spread of the poliovirus remained a Public Health Emergency of International Concern. For example, the U.S. CDC identified polio outbreaks and poliovirus detections in 39 countries in 2025.
To address these concerns, a recent study highlighted the benefits of an innovative polio vaccine candidate.
According to a study published by NPJ Vaccines on March 31, 2025, the success of the poliovirus (PV) vaccines has enabled the near-eradication of wild PV. Their continued post-eradication use poses concerns due to the 'potential for virus escape during vaccine manufacture.'
While the current generation of PV vaccines has achieved great success, the continued use of oral PV has facilitated the continued appearance of circulating vaccine-derived PV (cVDPV), which now outnumbers wild PV cases yearly.
These researchers wrote, 'Recombinant virus-like particles (VLPs) that lack the viral genome remove this risk.'
They demonstrate the production of PV VLPs for all three serotypes by controlled fermentation using Pichia pastoris.
The cryo-EM structure of a new PV2 mutant, SC5a, was determined compared to PV2-SC6 b VLPs described previously, and the immunogenicity of PV2-SC5a VLPs was investigated.
Finally, a trivalent immunogenicity trial using bioreactor-derived VLPs of all three serotypes in the presence of Alhydrogel adjuvant showed that these VLPs outperform the current IPV vaccine in the standard vaccine potency assay, offering the potential for dose-sparing.
Overall, 'these results provide further evidence that yeast-produced VLPs have the potential to be a next-generation polio vaccine in a post-eradication world.'
'The most important pre-requisite of any next-generation PV vaccine is that it elicits the same long-lasting immunity against disease as the current vaccine.'
Over the past few years, PT Biofarma has produced the type 2 novel oral polio (nOPV2) vaccine, derived from the live, infectious virus and 'triple-locked.' The nOPV2 vaccine is reported to be more genetically stable than previous oral polio vaccines, with a lower risk of reversion to neurovirulence.
It has been administered over 1.1 billion times, mainly in Africa.
Since 2000, the IPV has been the standard polio vaccine offered in the United States, and booster doses are suggested when visiting poliovirus outbreak areas.

Recent data from the UK Health Security Agency (UKHSA) show that the number of imported Zika cases in England, Wales, and Northern Ireland (EWNI) increased last year.
On March 27, 2025, the UKHSA disclosed that the number of Zika virus disease cases reached 16 in England, Wales, and Northern Ireland during 2024, compared to 8 cases in 2023.
Most Zika-infected travellers returned to EWNI from South-Eastern Asia, where countries reported locally acquired infections.
For example, since 2016, the Zika virus has been reported in India's 16 different states/union territories.
Dr. Philip Veal, Consultant in Public Health at the UKHSA, stated in a media release, "It is essential to take precautions against mosquito-borne infections such as dengue while travelling abroad. Simple steps, such as using insect repellent, covering exposed skin, and sleeping under insecticide-treated bed nets, can reduce the risk of mosquito-borne infections."
Although Zika virus cases are rarely reported and don't often cause serious illness, the infection poses a significant risk to pregnant women, as it can be passed to the fetus.
An Original Investigation published by JAMA Public Health in January 2025 found that children born with congenital Zika syndrome (CZS) had a 13.10-fold higher hazard of death compared with those without CZS.
This health risk is well-known in the Region of the Americas, where Zika infections have substantially increased.
Over 42,127 ZIka cases were confirmed in the Americas in 2024, with the highest proportion of Zika cases reported in Argentina, Brazil, Bolivia, Colombia, and Costa Rica.
The U.S. CDC says Zika-spreading mosquitoes are found throughout Puerto Rico, where the Department of Health reported 16 cases in 2024.
In 2023, over 37,659 Zika cases were reported by various countries in the Americas.
As of March 31, 2025, no drug or vaccine prevents Zika virus infection. However, Zika vaccine candidates are conducting clinical trials in 2025.

Nipah virus, one of the deadliest pathogens known to infect humans, lacks a preventive vaccine. To accelerate the development of a Nipah vaccine candidate, Coalition for Epidemic Preparedness Innovations (CEPI) provides up to $13.38 million in funding for a pioneering self-amplifying mRNA (saRNA) vaccine candidate.
Gennova Biopharmaceuticals Limited developed this innovative solution and is teaming up with Houston Methodist Research Institute, a Texas-based institution.
They will use their cutting-edge AI technology to optimise the properties of proteins derived from the virus that could stimulate the immune system and serve as optimal vaccine targets for Gennova to investigate.
“Wi"h no vaccines or specific therapeutics approved for human use against Nipah, CEPI is leading the charge to protect the world against this deadly virus committing over $100 million to its Nipah programmes and advancing the first ever Nipah vaccine candidates into Phase 1 studies and through to completion," said Dr. Kent Kester, Executive Director of Vaccine Research and Development at CEPI, in a press release on March 30, 2025.
“Gennova will not only help establish the suitability of the saRNA platform for use against Nipah but also its suitability as part of a wider group of RNA technologies that could enable rapid responses to future Disease X threats, potentially within 100 days of identification.”
I" August 2023, CEPI initially provided up to $3.6 million to support the optimization of Gennova' sRNA-platform technology to develop vaccine candidates against unknown pathogenic threats known as Disease X.
The initial tranche of funding was part of CEPI's program to support novel RNA vaccine platform technologies for emerging and select endemic infectious diseases. These technologies could offer substantial advantages over existing mRNA technologies, such as multivalency, improved immunogenicity, storage and stability, productivity, response time, and cost-of-goods.
To help guide a path forward, the WHO's roadmap for Nipah virus research priorities (2024–29) outlines ambitious milestones for advancing diagnostics, therapeutics, and vaccines.
As of March 31, 2025, Nipah outbreaks have been confined to South and Southeast Asia. However, the fruit bat vector is found in large geographical areas across the globe, covering a population of more than 2 billion people.
According to the U.S. CDC, around 40%–70% of people infected with the Nipah virus may die.
Over the years, Ontario's measles cases have been rare, owing to the high immunization coverage. However, that favorable situation has changed in Canada's most populous province.
This new report from the public health ministry describes the epidemiology of measles in Ontario, focusing on the current multi-jurisdictional measles outbreak.
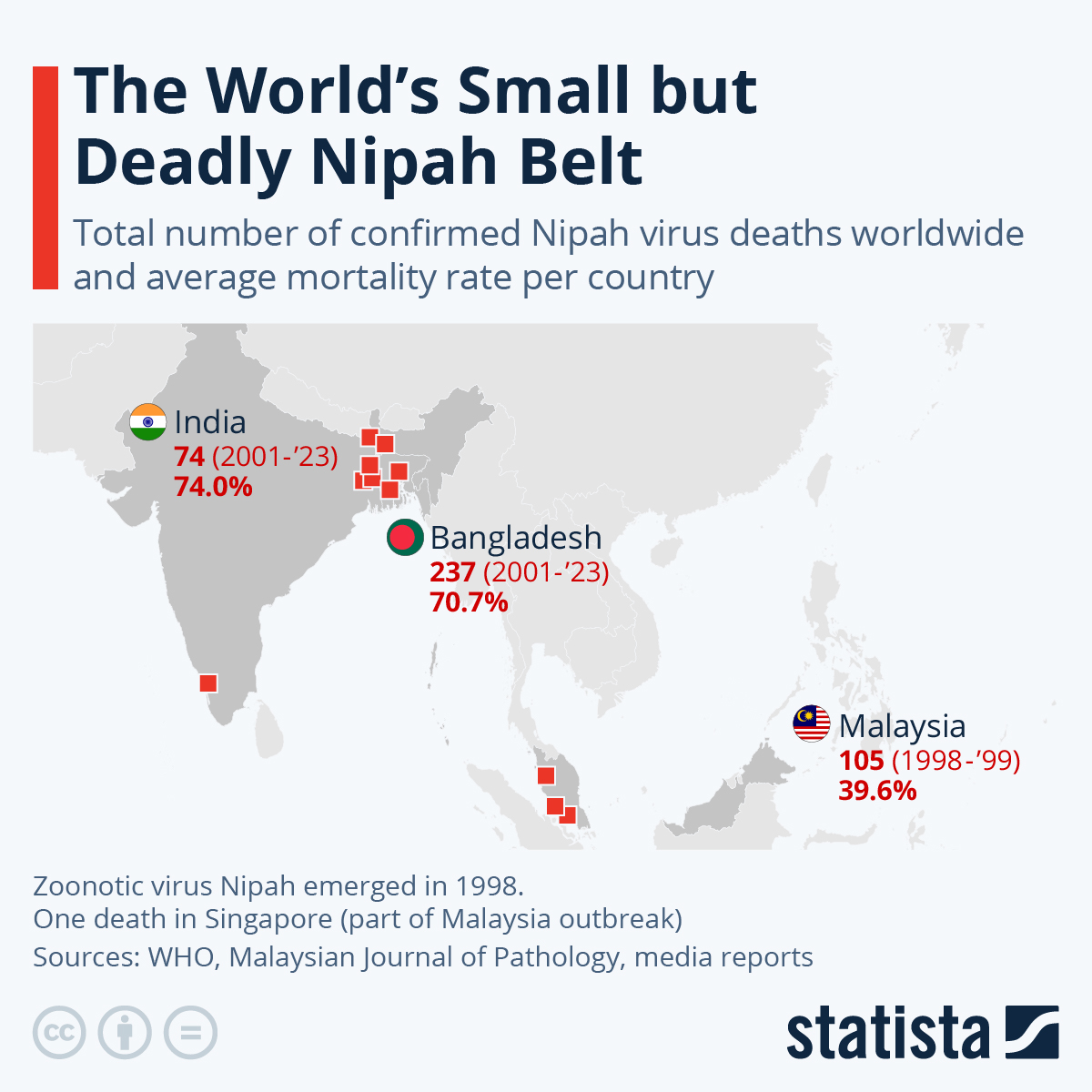
As of March 26, 2025, 557 measles cases (464 confirmed, 93 probable) had been reported in Ontario since October 28, 2024.
This is a significant change from the past decade.
Between 2013 and 2023, 101 confirmed measles cases were reported in Ontario, while 64 cases were reported in 2024.
Just to the south in the United States, Minnesota, Michigan, Ohio, Pennsylvania, and New York have also reported measles cases, but on a far reduced scale.
In 2025, a total 483 confirmed measles cases were reported by 20 U.S. jurisdictions.
As of March 30, 2025, the U.S. Centers for Disease Control and Prevention maintains a global measles outbreak Watch-Level 1, Notice, which does not include Canada.
The CDC recommends that most people get two doses of the MMR vaccine to protect against this infectious disease. Measles vaccines are generally available at travel clinics and pharmacies in the U.S.