Search API
The U.S. Centers for Disease Control and Prevention (CDC) published Key Updates for Week #2, ending January 14, 2023. This CDC report highlights both good and unfortunate news.
The Weekly U.S. Influenza Surveillance Report says seasonal influenza activity continues to decline across the U.S., with three regions below their outpatient respiratory illness baselines for the first time since October 2022.
And the majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season’s influenza vaccine, which remain available at most clinics and pharmacies in the U.S.
Furthermore, the National Center for Health Statistics Mortality Surveillance data available on January 19, 2023 shows that overall flu-related fatalities have decreased for the past four weeks.
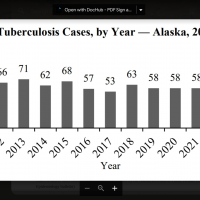
During week #2, there were 2,954 pneumonia, influenza, and/or COVID-19 (PIC) deaths.
Among those PIC deaths, 1,422 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, 1,281 documented pneumonia, and 251 listed influenza.
Unfortunately, the CDC also confirmed six additional influenza-associated pediatric fatalities have occurred during the 2022-23 flu season. This news increases the total of 85 pediatric flu deaths reported so far this season.
During the last flu season, there were only 45 pediatric fatalities related to the flu.
The CDC says an annual flu shot remains the best way to protect against influenza infections and can also prevent serious outcomes in people who get vaccinated but still get sick with the flu.
CDC recommends that everyone ages six months and older get an annual flu vaccine as long as flu activity continues, which could be several additional months.
So far this flu season, 171.52 million doses have been distributed in the U.S.
HUTCHMED Limited today announced it entered into an exclusive license agreement with a subsidiary of Takeda Pharmaceutical Company Limited to further the global development, commercialization, and manufacture of fruquintinib.
Fruquintinib is orally administered and has the potential to be used across subtypes of metastatic colorectal cancer (“CRC”), regardless of biomarker status.
It is a highly selective and potent inhibitor of vascular endothelial growth factor receptors -1, -2, and -3.
CRC is a type of cancer that starts in either the colon or rectum.
Although early-stage CRC can be surgically resected, metastatic CRC remains an area of high unmet need with poor outcomes and limited treatment options.
HUTCHMED confirmed on January 23, 2023, it will receive up to US$1.13 billion, including US$400 million upfront on closing, as well as potential regulatory, development, and commercial sales milestone payments, plus royalties on net sales.
“Fruquintinib has the potential to change the treatment landscape for patients with refractory metastatic CRC who need additional treatment options. We look forward to utilizing our development and commercial capabilities to expand the potential of this innovative medicine to patients beyond China,” commented Teresa Bitetti, President of the Global Oncology Business Unit at Takeda, in a related press release.
Positive results of FRESCO-2, the global Phase III multi-regional clinical trial of fruquintinib in refractory metastatic CRC, were presented at the European Society for Medical Oncology Congress in September 2022. FRESCO-2 met its primary endpoint of improving overall survival in patients with metastatic CRC and was generally well tolerated.
According to the International Agency for Research on Cancer, CRC is the third most prevalent cancer worldwide, associated with more than 935,000 deaths in 2020.
In the U.S., an estimated 155,000 patients were diagnosed with CRC, and there were 54,000 related fatalities.
HUTCHMED stated it would continue to focus on progressing late-stage clinical trials and the commercialization of fruquintinib in mainland China in collaboration with Eli Lilly and Company, where it is approved under the brand name ELUNATE® for the treatment of patients with metastatic CRC who have been previously treated with fluoropyrimidine, oxaliplatin, and irinotecan, including those who have previously received anti-vascular endothelial growth factor therapy and/or anti-epidermal growth factor receptor therapy (RAS wild type).
ELUNATE has been included in the China National Reimbursement Drug List since January 2020 and was commercially launched in China in November 2018.

Genexine recently announced it received Fast Track Designation from the Korean Ministry of Food and Drug Safety (MFDS) for GX-188E, its first-in-class proprietary therapeutic DNA vaccine targeting advanced cervical cancer.
Following an evaluation of Phase 2 data from the recently completed clinical trial in advanced cervical cancer, Korea’s Health Authority (MFDS) concluded that GX-188E met the criteria for fast-track designation.
Genexine recently reported Phase 2 trial data which evaluated the efficacy and safety of the combination of GX-188E and KEYTRUDA®, anti-PD-1 therapy, in a total of 65 patients with HPV 16- and/or HPV 18- positive recurrent or metastatic advanced cervical cancer.
The final efficacy analysis evaluated in 60 patients showed an Objective Response Rate of 35%, indicating that of the 60 patients with advanced cervical cancer, 21 patients saw either over 30% reduction in tumor size or complete remission.
“We are grateful to the MFDS for their careful evaluation and recognition that GX-188E has the potential to be a key life-saving drug for the treatment of advanced cervical cancer,” said Neil Warma, Genexine’s President and CEO, in a press release on January 20, 2023.
“We are committed to the cancer patients in which this therapy could be effective..... We are designing the optimal Phase 3 study with GX-188E and expect to initiate that study this year (2023).”
HPV prevention vaccines are generally available at clinics and pharmacies worldwide.

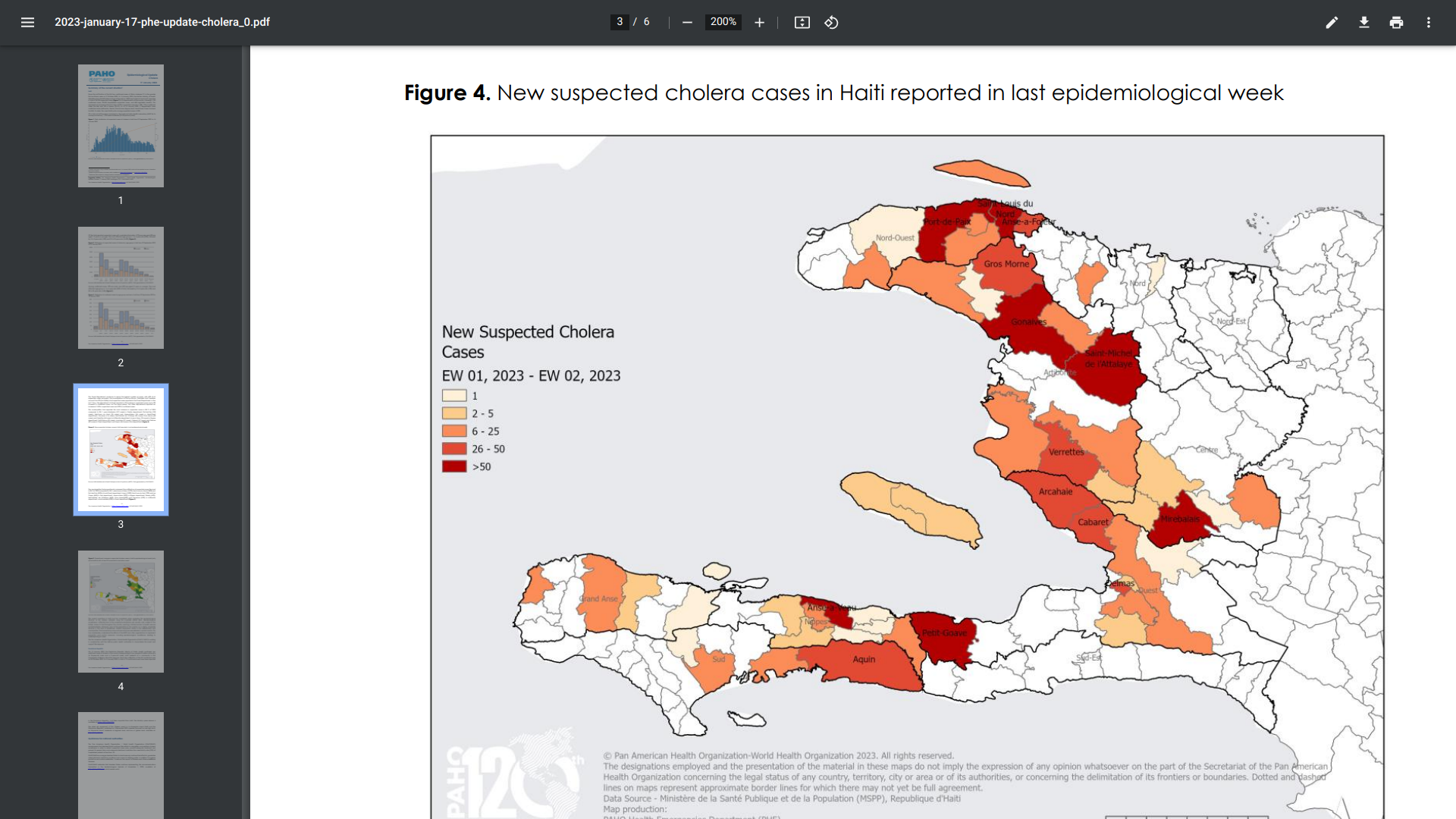
The Pan American Health Organization (PAHO) recently reported that since the initial cholera cases (Vibrio cholerae O1) in the greater Port-au-Prince area in October 2022, the Haitian Ministry of Health has reported a total of 24,232 suspected cases and 483 registered fatalities.
As of January 14, 2023, the Ouest Department, which includes the municipalities of Port-au-Prince, Cité-Soleil, and Carrefour, continues to report the highest number of cases, with 67% (N=10,836) of all suspected cases reported.
During a similar time frame, a total of 19 confirmed cases have been reported in the Dominican Republic (DR), with five of them imported from Haiti.
In a press release on January 15, 2023, the DR's Ministry of Public Health urged residents not to be alarmed and to remain attentive to the issued reports.
The latest PAHO risk assessment of the Cholera event in La Hispaniola Island (Haiti and the Dominican Republic) assesses the event as very high risk locally, moderate at the regional level, and low at the global level.
The U.S. Centers for Disease Control and Prevention (CDC) stated in 2022, vaccination may be considered for children and adults traveling to areas of active cholera transmission.
As of January 2023, cholera vaccines remain unavailable in the U.S.
Cholera is rare in travelers but can be severe. Certain factors may increase the risk of getting cholera or having severe disease. The CDC says avoiding unsafe food and water and washing your hands can also help prevent cholera.
Furthermore, the U.S. Department of Stated announced in December 2022 do not travel to Haiti due to civil unrest. U.S. citizens should depart Haiti now in light of the current security and health situation and infrastructure challenges.
Furthermore, clinicians should be prepared to treat cholera cases in travelers returning to the U.S. in 2023.
New Zealand's pending Prime Minister recently reconfirmed that the local vaccination policy requiring everyone to be fully vaccinated against COVID-19 will continue in 2023.
Chris "Chippy" Hipkins, the current minister of education, police, and Public Service, stated in an earlier video, '... if you haven't been vaccinated, you will be identified.'
According to a Bloomberg report on January 21, 2023, the former COVID minister will soon become New Zealand's prime minister.
This vaccination philosophy is synergistic with the U.S. Centers for Disease Control and Prevention (CDC) November 2022 statement that all eligible travelers to New Zealand should be up to date with their COVID-19 vaccines.
Furthermore, the CDC suggests international travelers should ensure they are up-to-date on all routine vaccines before every trip abroad in 2023.
This suggestion includes measles vaccinations.
New Zealand's 2019–2020 measles outbreak was an epidemic that primarily impacted the Auckland region.
To alert travelers, the CDC published a Watch-Level 1, Practice Usual Precautions notice regarding measles as an ongoing risk worldwide on December 1, 2022.
Furthermore, New Zealand's northern neighbor of India is confronting an ongoing measles outbreak in 2023, with over 12,000 cases impacting various areas last year.
The U.S. Department of State recently updated it's Level 2: Exercise Increased Caution for when visiting the southernmost continent of Antarctica.
As of January 19, 2023, the State Department announced travelers should be aware of environmental hazards posed by extreme and unpredictable weather and limited emergency services.
For travelers to Antarctica, severe low temperatures and high winds are the primary health hazards.
Temperature patterns vary widely because Antarctica is covered in continuous darkness during the winter and constant sunlight during the summer.
The sun's effects in Antarctica can damage the eyes and skin, and protective measures should be taken.
The U.S. Centers for Disease Control and Prevention (CDC) suggests various travel vaccinations, such as measles, before visiting Antarctica.
And if you travel to Antarctica, obtain comprehensive travel, medical, and medical evacuation insurance; see our webpage for more information on insurance providers for overseas coverage, says the State Department.
The U.S. government does not maintain an embassy or consulate in Antarctica.
If you need U.S. consular services when in Antarctica, contact the closest U.S Embassies/Consulates in Argentina, Australia, Chile, New Zealand, and South Africa.
From outside the U.S., call 1-202-501-4444 to speak with a U.S. representative.
Furthermore, government resources in the Antarctic Region are committed to the Antarctic Program (USAP).
Private expeditions should be self-sufficient and are encouraged to carry adequate insurance coverage against the risk of incurring financial charges or material losses while in the Antarctic.
The National Science Foundation, as manager of the USAP, reserves the right to seek, in accordance with international and domestic law, recovery of all direct and indirect costs of any such emergency search and rescue.
The Antarctic also includes island territories within the Antarctic Convergence, says the National Geographic Society.
Most of the islands and archipelagos of Lesser Antarctica are volcanic and heavily glaciated.
The islands of the Antarctic region are the South Orkney Islands, South Shetland Islands, South Georgia, and the South Sandwich Islands, all claimed by the United Kingdom.
Peter I Island and Bouvet Island, claimed by Norway.
Heard and McDonald islands, claimed by Australia.
And Scott Island and the Balleny Islands, claimed by New Zealand.