Search API
AC Immune SA today announced the first interim safety, tolerability, and immunogenicity findings from the Phase 1b/2 ABATE trial of its anti-amyloid-beta (Abeta) vaccine ACI-24.060 in patients with prodromal Alzheimer's disease (AD).
Early results from the first cohort of AD patients in ABATE showed that low dose ACI-24.060 could elicit an anti-Abeta antibody response as soon as week 6 (2 weeks after the second injection).
The data show that ACI-24.060 vaccination has been safe and well tolerated to date.
As a result, dosing in ABATE's second, higher-dose AD cohort has begun, and the trial is cleared to start screening specific individuals for part 2 of the study.
Dr. Andrea Pfeifer, CEO of AC Immune SA, commented in a press release on January 26, 2023, "We are delighted with the encouraging initial safety, and immunogenicity findings for ACI-24.060 in ABATE reported today."
"We believe ACI-24.060's successful development could provide patients with a novel therapeutic option offering numerous potential advantages in treatment, maintenance, and prevention settings."
"These early findings from ABATE represent an important step towards this goal, and we look forward to reporting more detailed data at a future conference."
ACI-24.060, derived from AC Immune's SupraAntigen® platform, has been shown in preclinical studies to induce a strong polyclonal antibody response that matures and is maintained against oligomeric and pyroglutamate-Abeta species, essential pathological forms of Abeta believed to drive Abeta plaque formation and disease progression.
Targeting Abeta using antibodies has recently been validated with U.S. FDA approvals of new monoclonal antibody treatments for patients with AD.
By eliciting polyclonal anti-Abeta antibodies, the ACI-24.060 anti-Abeta vaccine development program aims to ultimately deliver significant benefits to patients, their caregivers, and healthcare systems regarding potential safety and tolerability, low-frequency dosing, low overall costs, and durable responses.

TG Therapeutics, Inc. today announced the commercial launch of BRIUMVI™, for treating relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
BRIUMVI is the first and only anti-CD20 monoclonal antibody (mAbs) approved for patients with RMS that can be administered in a one-hour infusion following the starting dose.
BRIUMVI targets a unique epitope on CD20-expressing B-cells and was designed to lack specific sugar molecules normally expressed on the antibody.
Removing these sugar molecules, a process called glycoengineering allows for efficient B-cell depletion at low doses.
BRIUMVI was approved by the U.S. Food & Drug Administration based on data from Phase 3 trials, which demonstrated superiority over teriflunomide in significantly reducing the annualized relapse rate (the primary endpoint), the number of T1 Gd-enhancing lesions and the number of new or enlarging T2 lesions.
Michael S. Weiss, the Company's Chairman and CEO stated in a press release on January 26, 2023, "Our team has been working hard to make BRIUMVI available as quickly as possible, and we are pleased to announce that BRIUMVI is now available to healthcare providers and patients."
"We continue to be highly focused on ensuring patients who benefit from BRIUMVI can easily access treatment."
RMS is a chronic demyelinating disease of the central nervous system. It includes people with relapsing-remitting multiple sclerosis and people with secondary progressive multiple sclerosis who continue to experience relapses.
The results from the ULTIMATE I & II trials were published on August 25, 2022, in The New England Journal of Medicine.
The launch of BRIUMVI includes an extensive patient support program designed to support patients through their treatment journey. More information about the BRIUMVI Patient Support program can be accessed at www.briumvi.com.
TG Therapeutics was founded with one goal in mind—to leverage scientific advances in B-cell biology to develop novel treatments for patients.

A clinical-stage biotechnology company announced today good news regarding its Zika virus (ZIKV) vaccine candidate, which was recently found very effective in a preclinical study.
The U.S. Patent and Trademark Office (USPTO) issued GeoVax Labs Inc. a Notice of Allowance for Patent Application No. 17/000,768 titled, "Method for Generating a ZIKV Immune Response Utilizing a Recombinant Modified Vaccinia Ankara Vector Encoding the NS1 Protein."
The claims to be granted in the patent cover GeoVax's MVA vector comprising a nucleic acid sequence encoding a ZIKV nonstructural (NS1) protein, of which the GEO-ZM02 vaccine candidate is designed.
"Our novel Zika vaccine, GEO-ZM02, is constructed using our modified vaccinia Ankara (MVA) vector platform," stated GeoVax CEO David Dodd in a press release on January 25, 2023.
"Preclinical studies demonstrated a single dose of GEO-ZM02 provided 100% protection against a lethal dose of Zika virus."
"Addressing many of the world's most threatening infectious diseases is part of our vision and corporate priorities for MVA's applications, including an MVA-based next-generation COVID-19 vaccine currently in Phase 2 clinical trials."
With an outstanding safety record, MVA has great potential to address the unmet need to vaccinate women of childbearing age and newborns against ZIKV.
A pathogen endemic in various areas of the world, ZIKV is linked to an increase in infant microcephaly and neurodegenerative disease, Guillain-Barre syndrome, in adults.
Numerous public health officials recommend avoiding exposure to ZIKV, delaying pregnancy, and following basic supportive care (fluids, rest, and acetaminophen) after infection.
GEO-ZM02 is designed to function through the induction of T-cell responses rather than antibodies to eliminate the risk of Antibody Dependent Enhancement, a serious side effect observed in flavivirus infections when an individual does not have a fully protective immune response from vaccination or a previous infection that causes a more serious disease if infected.
ZIKV is a member of the Flaviviridae family, which includes other significant pathogens such as dengue, yellow fever, Japanese encephalitis, tick-borne encephalitis, and West Nile viruses.
GeoVax Labs, Inc. is developing novel therapies and vaccines for cancers and many of the world's most threatening infectious diseases.
As of January 25, 2023, the U.S. FDA has not approved a Zika prevention vaccine.
Note: The USPTO provides inventors, entrepreneurs, and small businesses with free resources on how to protect their intellectual property.

EverGlade Consulting today announced that Sabin Vaccine Institute successfully secured up to $214 million in funding from the U.S. government to advance the development and production of single-dose vaccine candidates for Ebola Sudan and Marburg virus diseases.
Currently, no licensed vaccines against either virus cause hemorrhagic fever and kill approximately half the people infected.
There are U.S. Food and Drug Administration-approved vaccines for a different ebolavirus known as Zaire.
BARDA, part of the U.S. Department of Health and Human Services' Administration for Strategic Preparedness and Response, will initially invest $35 million to produce up to 100,000 doses of the ChAd3-SUDV Ebola Sudan virus vaccine candidate.
These vaccines may be part of ongoing U.S. preparedness efforts and response to future global outbreaks.
The contract also includes funding to manufacture Sabin's Marburg virus vaccine, ChAd3-MARV, which will generate doses that could be used in trials and response to a future Marburg virus outbreak.
Andrew Stiles, Principal at EverGlade, said in a press release on January 25, 2023, "The recent Ebola Sudan outbreak in Uganda emphasized the critical need for better preparedness."
The initial Ebola virus disease (EVD) case first appeared in 1976.
The recent Sudan Ebolavirus outbreak in the Republic of Uganda was declared on September 20, 2022, and was declared ended in early 2023.
Detailed ebola vaccine information is posted at PrecisionVaccinations.com/Ebola.

A recent measles case in Christian County, Kentucky, has been associated with Ohio’s ongoing measles outbreak, according to Kentucky Cabinet of Health and Family Services spokesman Brice Mitchell on January 20, 2023.
WAVE3.com reported Mitchell indicated several other Kentucky residents are being monitored for measles symptoms.
The U.S. Centers for Disease Control and Prevention (CDC) says measles symptoms appear 7 to 14 days after contact with the virus, and rashes appear 3 to 5 days after the first symptoms.
Measles isn’t just a little rash.
According to the CDC, measles can be dangerous, especially for young children.
The good news is measles is a vaccine-preventable disease.
Recently, the city of Louisville, Kentucky, and the Jefferson County Public Schools began conducting measles vaccination clinics for about 10,000 unvaccinated students.
On January 18, 2023, local media reported on-campus measles clinics at Iroquois High School, Marion C. Moore School, Newcomer Academy, and Fern Creek High School on February 7, 2023.
Since June 2022, the Health Department of the City of Columbas, Ohio, and Franklin County Public Health have reported (85) confirmed measles cases in children, of which (36) were hospitalized as of January 24, 2023.
The CDC reported there were 121 measles cases in six U.S. jurisdictions in 2022.
During 2021, a total of 49 measles cases were reported by five jurisdictions.
Measles outbreaks continue to be reported worldwide, which is why the CDC recommends being fully vaccinated before visiting measles-outbreak countries such as India and Nigeria.

Peru's Culture Ministry website recently posted a notice that it had closed the country's most famous tourist attraction "to protect the safety of tourists and the population in general."
As of January 21, 2023, the 15th-century ancient ruins of Machu Picchu were not accepting future visitors to this mountaintop site, as the train service was recently closed.
There are a number of hiking routes along the Inca Trail to reach Machu Picchu, however, it takes about five days to reach the top.
Traditionally, over 2,000 people visit Machu Picchu daily.
Machu Picchu is located in the Eastern Cordillera of southern Peru on a 2,430-meter mountain ridge.
Cusco, Peru, where Machu Picchu is located, has been the site of some civil unrest in 2023.
The local airport, Alejandro Velasco Astete, was closed recently.
But, flights are expected to resume at Arequipa's Alfredo Rodriguez Ballon International Airport on January 26, 2023. According to the U.S. Embassy Peru, U.S. citizens should contact the appropriate airline for flight rescheduling.
For emergencies involving American citizens in Peru, please email [email protected] or call +51-1-618-2000. And U.S. citizens are advised to register in STEP to receive alerts and messages from the U.S. Embassy in Lima.

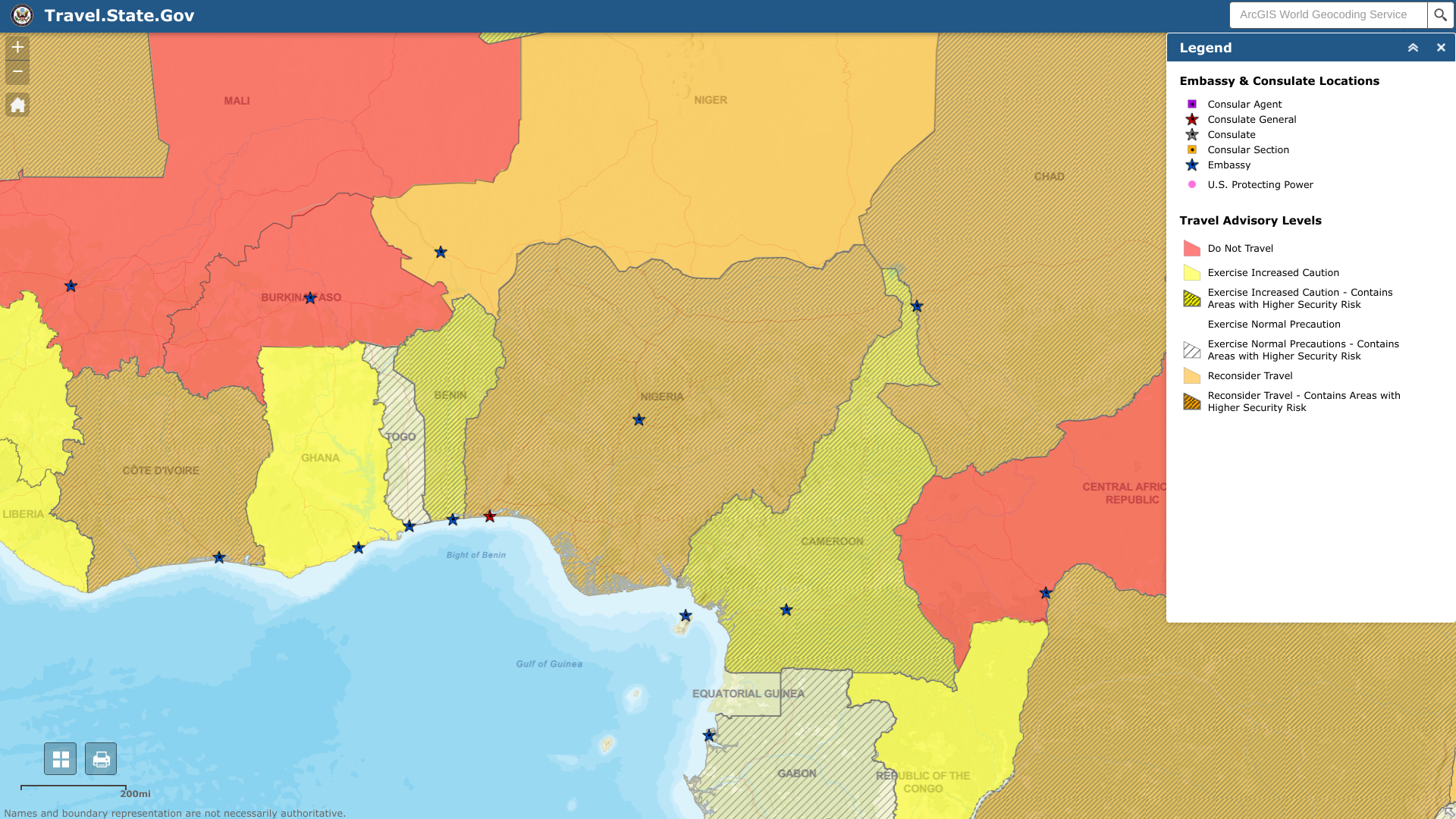
The U.S. Department of State updated its Level 3 Travel Advisory for the federal republic of Nigeria, located in west Africa.
Local media reported people should exercise caution around events in Lagos State from Jan. 23-24, 2023. And plan for localized transport disruptions.
As of January 23, 2023, the State Department says Do Not Travel to certain areas due to civil unrest:
- Borno, Yobe, Kogi, and northern Adamawa states,
- Bauchi, Gombe, Kaduna, Kano, Katsina, and Zamfara states,
- Coastal areas of Akwa Ibom, Bayelsa, Cross River, Delta, and Rivers states (except Port Harcourt).
Furthermore, the U.S. Consulate in Lagos provides all routine and emergency services to U.S. citizens in Nigeria. The U.S. Embassy Abuja can only provide emergency assistance to U.S. citizens in Abuja.
U.S. citizens in Nigeria who require assistance should contact [email protected] or +234 1 460 3410.
Additionally, they should enroll in the Smart Traveler Program to receive alerts and facilitate extraction during emergencies.
From a health perspective, the U.S. Centers for Disease Control and Prevention (CDC) advises future Nigeria visitors to speak with a travel vaccine advisory about one month before departure.
The CDC has recently issued various Travel Health notices for Nigeria's disease outbreaks, such as yellow fever, polio, measles, and Mpox.
The JAMA Network Open recently published an Original Investigation that found 41% of adults with impaired immune systems had received a 4th mRNA vaccine dose.
However, only 1% had received their recommended 5th dose
'Our results highlight a substantial gap in adherence to recommendations for mRNA monovalent COVID-19 booster doses,' wrote these researchers on January 20, 2023.
"Given the increased risk for severe COVID-19 in this vulnerable population and the well-established additional protection afforded by booster doses, targeted and tailored efforts to ensure that immunocompromised individuals remain up to date with COVID-19 booster dose recommendations are warranted," the researchers added.
Immunocompromised individuals (i.e., persons with immunocompromising conditions or who are taking immunosuppressive medications) often mount weaker immune responses to vaccines and experience higher rates of vaccine failure compared with immunocompetent individuals.
As of January 2023, both the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention have progressively amended COVID-19 vaccine authorizations and recommendations to include additional COVID-19 vaccine doses for immunocompromised individuals.
Disclosures: Pfizer, a company producing mRNA vaccines, sponsored his study. Corresponding Author: Sara Y. Tartof, Ph.D., MPH, Department of Research and Evaluation, Kaiser Permanente Southern California ([email protected]).


