Search API
The state of Florida's Week#4 Arbovirus Surveillance report disclosed no new locally-acquired dengue cases had been confirmed in 2023.
However, there were 68 cases of locally acquired dengue last year.
And Florida confirmed on January 28, 2023, international travelers continue returning to Florida infected with dengue.
So far, in 2023, 14 travel-associated dengue fever cases have been reported.
Last year, 871 travel-associated dengue cases were reported in Florida.
Since most of these dengue cases have been found in south Florida, the Florida Department of Health in Miami-Dade County issued a mosquito-borne illness advisory during the summer of 2022.
The U.S. Centers for Disease Control and Prevention says dengue is a vaccine-preventable disease. However, dengue vaccination in the U.S. with Dengvaxia requires pre-administration testing and counseling.

The Columbus City Health Department tweeted today that the current measles outbreak in central Ohio reached 42 days or two incubation periods since the last rash onset in a confirmed case.
This milestone fits the U.S. CDC's definition of the end of an outbreak; however, 'we still have test results pending for suspected cases.'
Measles outbreaks are declared when the number of cases reported in an area is higher than the expected number of cases.
Around 90% of people who are not protected will become infected following exposure to the measles virus.
As of February 3, 2023, the City of Columbus, Ohio, reported 85 measles cases since the initial cases were reportedpotentially connectedthese most unvaccinated children had been hospitalized.
Throughout the U.S., the CDC has reported two measles cases in 2 jurisdictions in 2023.
One of these new measles cases was reported in Kentucky, with a potential connection to the Ohio outbreak.
In response, the Jefferson County Public Schools, located near Lousiville, Kentucky, began conducting measles vaccination clinics at schools for about 10,000 under-vaccinated students.
The CDC reported on January 13, 2023, nationally, the 2-dose MMR coverage was 93.5% during the 2020–2021 school year.
Measles vaccines are generally available at clinics and pharmacies in the U.S.

The Ministry of Health and Family Welfare, Government of the People's Republic of Bangladesh, has marked 32 districts at risk of the Nipah virus infection and has identified ten cases in 2023.
According to local media on February 4, 2023, the Dhaka North City Corporation's hospital in Mohakhali has also been instructed to prepare 20 beds for treating Nipah virus patients.
"This is because anywhere a Nipah virus patient is also likely to have bats and palm sap. Therefore, all parts of the country are vulnerable to the Nipah virus, but the districts where it has been detected are advised to take extra precautions."
The World Health Organization says Nipah virus infection is a zoonotic illness transmitted from animals, through contaminated food, or from person to person.
As of February 5, 2023, the U.S. FDA has not approved a Nipah virus preventive vaccine candidate.
However, The Lancet published results from a vaccine candidate study in December 2022 that concluded the VSV-NiVG vaccine offers broad protection against NiV disease.
The U.S. CDC does suggest various vaccinations, including for dengue, before visiting Bangladesh in 2023.
The Canadian government recently confirmed visitors to Buenos Aires, Argentina, should exercise high caution due to civil unrest.
As of January 31, 2023, Canada stated when in the Buenos Aires tourist areas of La Boca, Congreso, Florida Street, the Retiro bus station area, and San Telmo, be aware of your surroundings.
And in La Boca, always remain on Caminito Street, as thefts often occur on neighboring streets, and avoid the area after dark.
Furthermore, since 2019, several incidents of tourists have been followed from Buenos Aires' Ministro Pistarini International Airport to their hotels and robbed. In some cases, the criminals responded violently when the victims resisted.
If you are the victim of a crime, call the local police at 911, as the U.S. Embassy cannot take custody of U.S. citizens or intervene in Argentine legal procedures. All U.S. citizens are subject to the laws of the Government of Argentina.
The Embassy suggests enrolling in the STEP program to receive important information about safety conditions, helping you make informed travel plans.
The U.S. Embassy in Buenos Aires is located at Av. Colombia 4300 (C1425GMN). Telephone: (54-11) 5777-4533.
From a health perspective, the U.S. Centers for Disease Control and Prevention (CDC) says there are no travel health notices in effect for Argentina as of February 5, 2023.
However, the CDC suggests various travel vaccinations, including yellow fever.
Yellow fever vaccination with either Stamaril or YF-Vax is recommended for travelers ≥9 months of age going to Corrientes and Misiones Provinces. And is not recommended for travelers going to Formosa Province and designated areas of Chaco, Jujuy, and Salta Provinces.
And the U.K.'s travel advisory for Argentina includes notices regarding dengue and Zika virus transmission.

Public Health recently announced it was extending negative COVID-19 test requirements for arriving air travelers from the Peoples' Republic of China.
Initially announced in early January 2023, this extension is scheduled to be a requirement until April 5, 2023.
This extension applies to travel to Canada through connecting flights from other countries, for example, if your flight itinerary starts in China and includes a connecting flight from Germany to Canada.
And those transiting through Canada with onward travel to another country.
If Canada isn't your final destination, also check the travel and testing requirements for your final destination.
However, this doesn't apply to travelers transiting through China, Hong Kong, or Macao, who are there for 24 hours or less.
For example, if your flight itinerary starts in Australia and includes a connecting flight in China before continuing to Canada, and if you are in China for 24 hours or less before the connecting flight to Canada is initially scheduled to depart.
And travelers must keep the proof of their test result until leaving the airport in Canada.
In the U.S., the Centers for Disease Control and Prevention issued an Alert - Level 2, Practice Enhanced Precautions, with similar requirements, on January 5, 2023.

The U.S. Food and Drug Administration (FDA) issued an updated Emergency Use Authorization (EUA) stating that the oral antiviral Paxlovid™ is now offered to people with a SARS-CoV-2 coronavirus infection without a confirmatory test.
On February 1, 2023, Patrizia Cavazzoni, M.D. Director Center for Drug Evaluation and Research with the FDA published EAU #105, confirming certain adults and pediatric patients can be prescribed Paxlovid without completing a diagnostic test.
However, there are known drug interactions that should be reviewed.
Pfizer previously confirmed Paxlovid is contraindicated in patients with a history of clinically significant hypersensitivity reactions to its active ingredients (nirmatrelvir or ritonavir) or any other components of the product and with drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions.
Therefore, before prescribing Paxlovid, clinicians should carefully review the patient's concomitant medications, including over-the-counter medicines, herbal supplements, and recreational drugs, says the U.S. NIH.
Pfizer Inc., Paxlovid's producer, stated on January 31, 2023, it expects a 58% reduction in related revenues in 2023.

The end of the Mosaico HIV vaccine trial in January 2023 must lead to a continued drive to innovate and an urgency to ensure that proven HIV prevention and treatment options reach all who need them stated UNAIDS.
Although no safety concerns were flagged during the HPX3002/HVTN706 clinical trial, it was discontinued after an independent review found no evidence of reduced risk of HIV infection among vaccinated participants.
The study evaluated an investigational vaccine regimen containing a mosaic-based adenovirus serotype 26 vector (Ad26.Mos4.HIV) administered during four vaccination visits over one year. In addition, a mix of soluble proteins (Clade C/Mosaic gp140, adjuvanted with aluminum phosphate) was also administered at visits three and four.
“The disappointment of the vaccine trial further underlines the importance of rolling out available HIV treatment and prevention innovations,” said UNAIDS Executive Director Winnie Byanyima in a press release on January 23, 2023.
“The search for a vaccine must continue, but it’s important to remember that despite this setback, the world can still end AIDS by 2030 by delivering all the proven prevention and treatment options to all the people who need them.
Rapid progress against the HIV pandemic is possible if existing prevention and treatment options are made available by sharing technologies, expanding provision, and tackling barriers to access.
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination, and zero AIDS-related deaths.
The U.S. White House National Mpox Response recently noted that approximately 40% of people diagnosed with mpox also had HIV. In 2022, about one million people were vaccinated against Mpox in the USA.
As of February 3, 2023, HIV vaccine candidates continue clinical research studies.

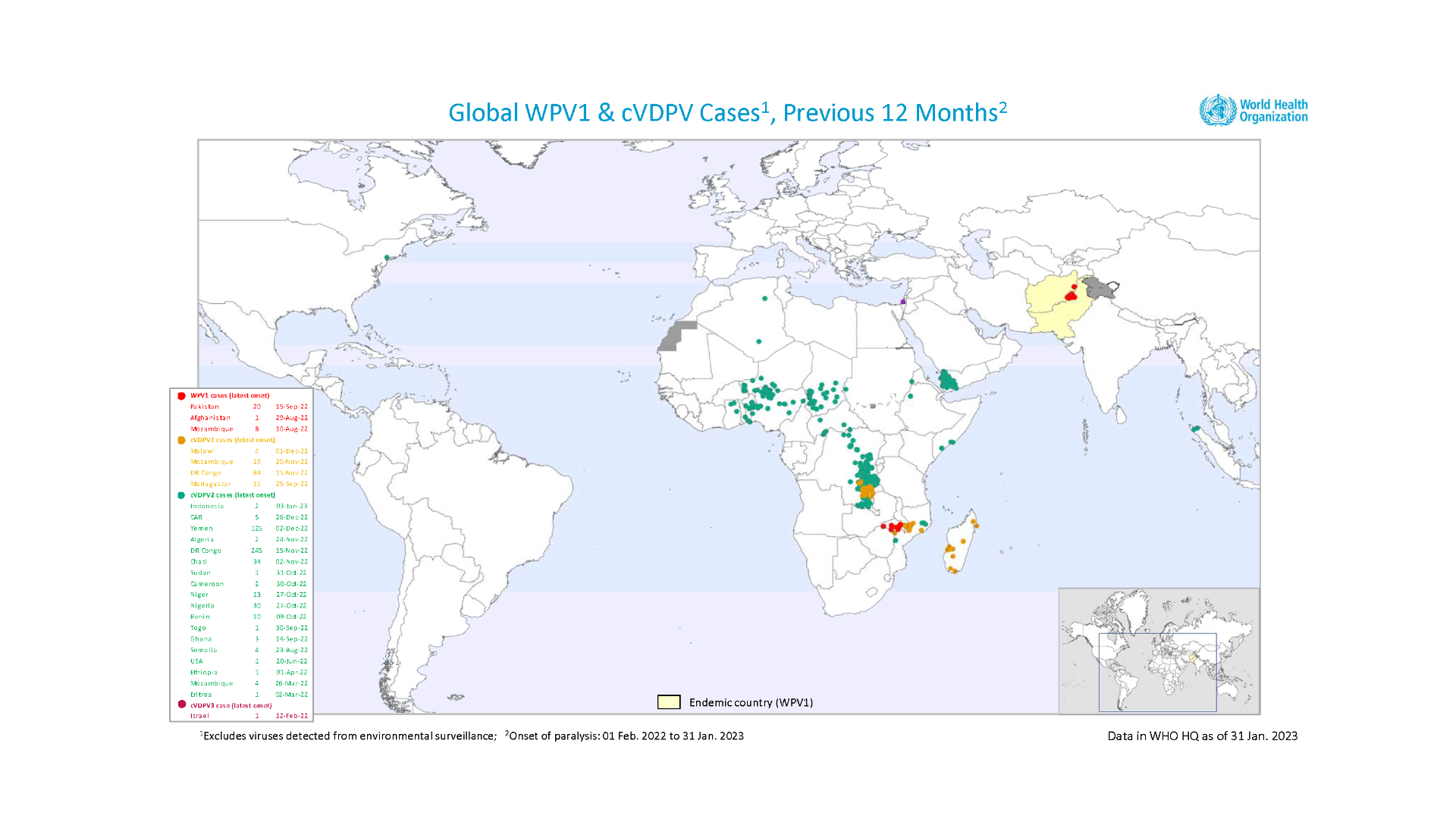
The World Health Organization (WHO) recently reaffirmed the spreading of poliovirus, and recent polio cases remain a public health emergency of international concern (PHEIC).
The WHO's Emergency Committee reviewed the data on wild poliovirus (WPV1) and circulating vaccine-derived polioviruses (cVDPV) in the context of the global target of eradicating WPV and cessation of outbreaks of cVDPV2 by the end of 2023.
Technical updates were received about the situation in the following countries: Afghanistan, Botswana, Canada, the Democratic Republic of the Congo, Indonesia, Madagascar, Nigeria, Pakistan, Sudan, and Zambia.
Although encouraged by the reported progress, the Committee unanimously agreed that the risk of the international spread of poliovirus remains a PHEIC and recommended the extension of Temporary Recommendations for a further three months into mid-2023.
The WHO Director-General endorsed the Committee's recommendations on February 1, 2023.
In the U.S., the Centers for Disease Control and Prevention (CDC) is leading the wastewater review for the continued spreading of poliovirus in New York, Michigan, and Pennsylvania in 2023.
The CDC stated in a Level 2 Travel Advisory posted on January 3, 2023, before traveling to any polio-risk destination, adults who previously completed the entire routine polio vaccine series receive a single, lifetime booster dose of polio vaccine.
Polio is a vaccine-preventable disease, says the CDC.
As a result, most clinics and pharmacies in the U.S. offer polio vaccination services in 2023.
According to reporting by Fierce Biotech, Merck Inc. confirmed on February 2, 2023, that it had discontinued the chikungunya vaccine candidate development program as part of a "routine pipeline prioritization."
While the vaccine candidate completed a phase 2 clinical trial, the study was suspended "due to a clinical stock recovery action," meaning it did not reach its original participant enrollment goal.
Merck's contender V184 was acquired through a $366 million takeover of Themis Inc. in 2020.
The chikungunya vaccine development race is led by Valneva SE, which completed the rolling submission of the VLA1553 vaccine's Biologics License Application to the U.S. Food and Drug Administration (FDA) in December 2022.
As of February 3, 2023, the FDA has not approved any chikungunya vaccine candidates.
This mosquito-transmitted disease remains a public health risk.
The Pan American Health Organization reported that in 2022, 87 fatalities were associated with chikungunya infections in the Americas last year.
Other chikungunya vaccine candidate news is posted at Vax-Before-Travel.com.