Search API
The Public Health Agency of Canada (PHAC) today issued a statement offering an update on the ongoing response to the Mpox outbreak.
On February 9, 2023, the World Health Organization (WHO) Director-General declared that Mpox remained a Public Health Emergency of International Concern.
This initial declaration was issued on July 23, 2022.
On February 14, 2023, the WHO noted a sustained decline in Mpox cases globally, with the majority of cases being reported from the Regions of the Americas, with 200-250 cases per week,
And about 4% of Mpox cases occurred in women.
Since the beginning of the Mpox outbreak, the Government of Canada has taken action to protect the health and safety of Canadians.
The ongoing management of Mpox relies on continued vigilance, re-emergence of cases, various public health measures, and vaccination.
Mpox vaccines will continue to be available in Canadian provinces and territories for those at higher risk, including second doses of Imvamune®.
Bavarian Nordic's MVA-BN vaccine is approved by the U.S. Food and Drug Administration for smallpox and Mpox under the JYNNEOS® brand.
Since May 2022, the Government of Canada has deployed over 145,000 doses of vaccine.
Mpox information can be found on the Government of Canada's Mpox: For health professionals website. Other outbreak news is posted at Mpox Today.

OSE Immunotherapeutics SA today provided a regulatory update on the clinical development plan of Tedopi®, a immunotherapy activating tumor-specific T-cells, in phase 3 in monotherapy in advanced or metastatic non-small cell lung cancer (NSCLC) after checkpoint inhibitor failure (ICI).
Nicolas Poirier, Chief Executive Officer of OSE Immunotherapeutics, commented in a press release on February 15, 2023, “We are pleased with the positive outcomes from the US Food & Drug Administration Type C Meeting following the supportive European Medicines Agency advice, as we are actively preparing a confirmatory phase 3 trial to support the regulatory registration of Tedopi®."
Both Agencies supported the continuation of the clinical development for Tedopi® through a new confirmatory phase 3 clinical trial versus standard of care in second-line treatment for HLA-A2+ patients in advanced in NSCLC.
Tedopi® is the first cancer vaccine to show positive and clinically meaningful efficacy results associated with a better safety and quality of life profile in monotherapy versus active comparator (chemotherapy-based standard of care) in the third line with secondary resistance to ICI in advanced or metastatic NSCLC.

The Nigeria Center for Disease Control and Prevention (NCDC) confirmed 216 diphtheria cases with 40 related fatalities deaths since December 2022.
About 184 diphtheria cases were reported as children, confirmed a news report on February 14, 2023.
The NCDC confirmed a previous diphtheria outbreak in Borno, north-eastern Nigeria, in 2011, with 98 cases and 21 deaths (the case-fatality ratio was 21.4%).
Diphtheria spreads quickly between people by direct contact or through the air through respiratory droplets from coughing or sneezing. It may also be spread by contaminated clothing and objects.
In Nigeria, Diphtheria infection is treated by administering a diphtheria antitoxin intravenously or through an intramuscular injection.
Antibiotics can also be given to eliminating the bacteria to prevent transmission and toxin production to others.
Children are fully vaccinated against diphtheria after three doses of the pentavalent vaccine, as the Nigerian immunization schedule recommends.
The latest U.K. travel advisory says serious tropical illnesses like malaria, typhoid, Lassa fever, and yellow fever occur in Nigeria.
And as of February 16, 2023, the U.S. CDC has issued various health alerts for Nigeria.

The U.S. National Center for Immunization and Respiratory Diseases today reported five additional children with Acute Hepatitis of Unknown Etiology were reported over the past month.
Investigators are examining a possible relationship to adenovirus type 41 infection, which is not a common cause of hepatitis in otherwise healthy children.
'While rare, children can still have serious hepatitis, and we don't always know the cause. That's why investigators continue to look at possible causes and investigate,' wrote the U.S. Centers for Disease Control and Prevention (CDC).
The U.S. CDC is looking broadly, including hepatitis cases of unknown origin in children under ten years of age.
Since October 1, 2021, the number of persons under investigation (PUI) has reached 389 as of February 15, 2023.
Among the reported PUIs, about 90% required hospitalization, a few needed a liver transplant, and over ten died.
The World Health Organization, the U.K., Europe, and Canada have recently reported acute liver inflammation infections of unknown etiology among children.

VBI Vaccines Inc. today announced interim data from the Phase 2 study evaluating the combination of VBI-2601 (BRII-179), VBI's HBV immunotherapeutic candidate, and BRII-835 (VIR-2218), an HBV-targeting siRNA candidate, in chronically infected HBV patients.
The data, which will be featured in an oral presentation on February 18, 2023, demonstrated that the combination therapy was generally well-tolerated, restored strong anti-HBsAg antibody responses, and led to improved HBsAg-specific T-cell responses when compared to BRII-835 alone.
Notably, in two participants who received the combination therapy, maximum reductions in HBsAg to an undetectable level or the lower limit of quantification (LLOQ) were achieved by Week 40, which were associated with robust HBV-specific antibody and T-cell responses.
Dr. Francisco Diaz-Mitoma, M.D., Ph.D., VBI's Chief Medical Officer, commented in a press release on February 15, 2023, "Numerous studies have assessed the potential of siRNA candidates in hundreds of chronically infected HBV patients, but this is the first time we've seen data from the combination of an HBV siRNA with an HBV-specific immunomodulatory."
"Consistent with the known mechanism of action of VBI-2601 and its inclusion of the pre-S1 and pre-S2 antigens in addition to the S antigen, these interim data indicate that VBI-2601 may be able to break tolerance to the S antigen, achieving immune restoration."
"Additionally, the reduction of S antigen to at or below the LLOQ, as seen in the two patients, is a noteworthy achievement in this population."
"We are very encouraged by these interim data, which suggest that the combination of VBI-2601 and an HBV siRNA has the potential to be a meaningful part of a functional cure regimen."
"We look forward to additional data from this study, as well as data from the ongoing Phase 2 study of VBI-2601 as an 'add-on' to existing pegylated interferon and nucleos(t)ide reverse transcriptase inhibitor therapy in non-cirrhotic chronic HBV patients, both of which are expected later this year."
An abstract summarizing the interim data is available at this link.

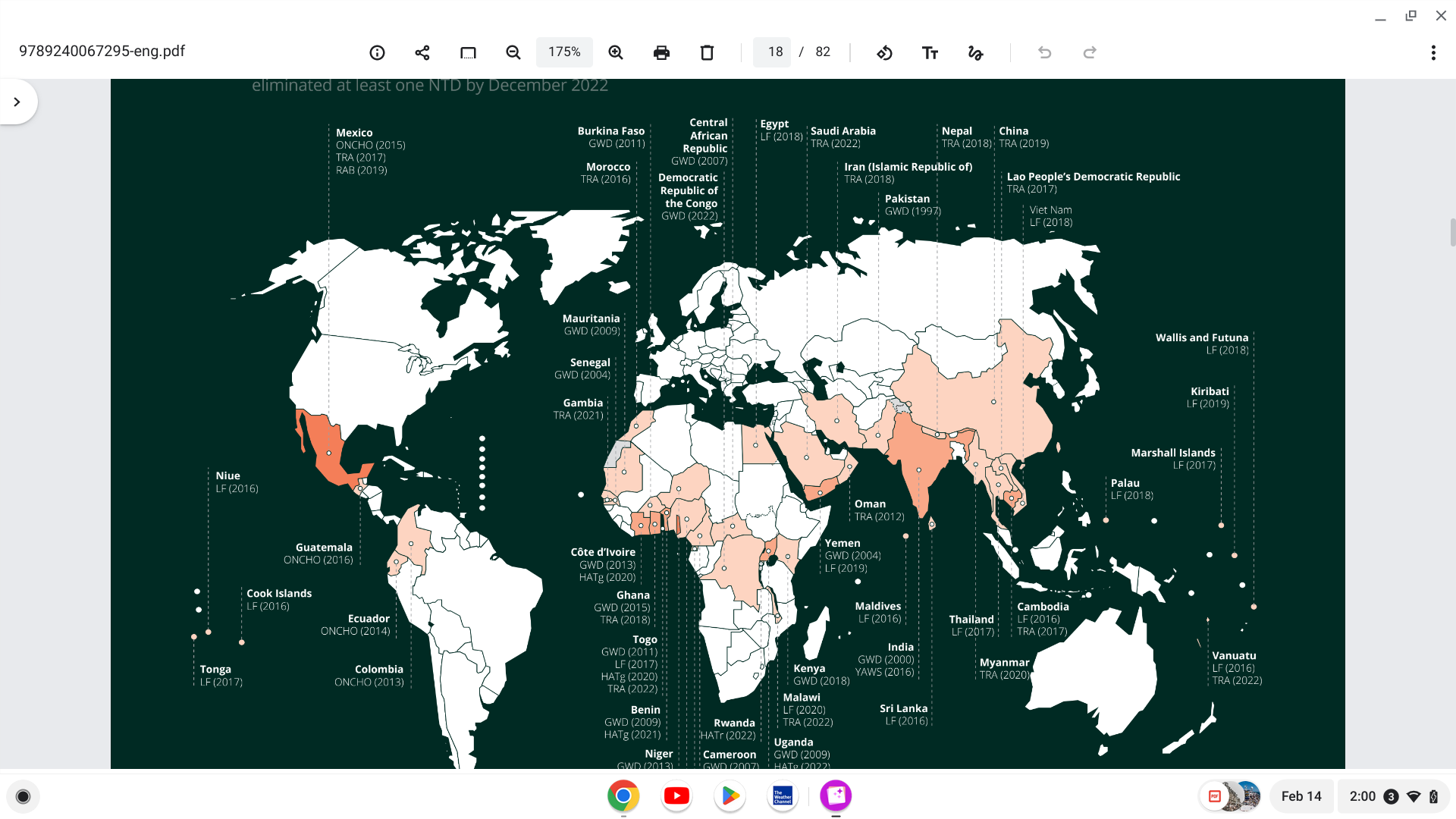
According to a recent World Health Organization (WHO) global report, 8 countries eliminated at least one neglected tropical disease last year. As of December 2022, 47 countries had eliminated at least one NTD.
Previously, more than 1 billion people were treated yearly for four consecutive years between 2016 and 2019.
But over the past decade, the number of people requiring interventions against NTDs has decreased by 25%.
And the burden of disease calculated in disability-adjusted life years is also steadily decreasing.
NTDs are a group of around 20 conditions that affect more than one billion people worldwide but have been largely overlooked by global health agendas.
The diseases generally affect people living in impoverished communities and can be caused by bacteria, viruses, fungi, parasites, or toxins.
The completed WHO Global Report (Jan. 2023) is posted at this link.
Vaxart, Inc. today announced that it had dosed the first subject in a Phase 2 dose-ranging clinical trial of its oral tablet bivalent vaccine candidate.
Vaxart expects to report topline data from the Phase 2 study in mid-2023.
"Initiating the Phase 2 clinical trial of this candidate is an important achievement toward our goal of developing an oral tablet vaccine that may reduce the significant global health threat that norovirus poses to children and seniors," said Dr. James F. Cummings, MD, Chief Medical Officer at Vaxart, in a press release on February 14, 2023.
"Results from the Phase 1b clinical trial in healthy adults demonstrate that this candidate stimulates robust IgA antibody-secreting cells against the prevalent strains of two norovirus genotypes that cause most norovirus disease."
"Data from the Phase 2 trial will inform our further clinical development strategy for this promising vaccine candidate."
As previously reported, Vaxart's bivalent vaccine candidate demonstrated robust immunogenicity, with an IgA ASC response rate of 78% for the GI.1 strain and 93% for the GII.4 strain, with no interference observed.
Three Phase 1 studies of Vaxart's norovirus pill vaccine indicated it is well tolerated and generated systemic and local immune responses that are both robust and persistent.
In the U.S., norovirus causes 21 million illnesses each year, infecting 15% of all children under the age of five years and resulting in illness — which frequently requires hospitalization — in 7.5% of people over 65 years.
Other norovirus vaccine candidate news is posted at PrecisionVaccinations.

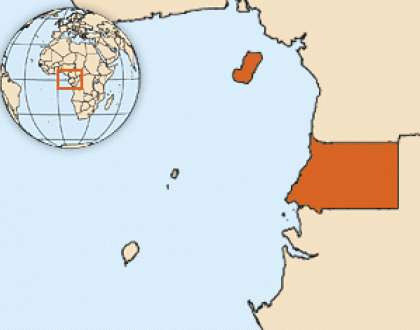
The World Health Organization (WHO) today announced it would convene an urgent Marburg virus vaccine consortium (MARVAC) meeting on February 14, 2023, to discuss the new Marburg virus disease outbreak in Equatorial Guinea, in Africa.
This is an open webinar, and non-MARVAC members can digitally attend at this Zoom link.
The MARVAC includes leaders in the field of vaccine research and development, working together to develop vaccines against this infectious disease threat.
As of February 14, 2023, no vaccines or antiviral treatments have been U.S. FDA-approved to treat this severe virus.
However, there are vaccine candidates currently being evaluated.
The emergence of the Marburg virus disease (MVD)in Africa triggered the assembly of the MARVAC consortium in 2022.
MVD is in the same virus family that causes Ebola virus disease.
This urgent action is related to yesterday's WHO announcement that Equatorial Guinea had confirmed its first-ever outbreak of Marburg virus disease.
Preliminary tests carried out following the deaths of at least nine people in the country's western Kie Ntem Province turned out positive for MARV viral hemorrhagic fever.
"Marburg is highly infectious. However, thanks to the rapid and decisive action by the Equatorial Guinean authorities in confirming the disease, emergency response can get to full steam quickly so that we save lives and halt the virus as soon as possible," commented Dr. Matshidiso Moeti, WHO Regional Director for Africa, in a press release.