Search API
Alzamend Neuro, Inc. today announced the completion of the clinical portion of its Phase IIA multiple ascending dose ("MAD") study for dementia related to Alzheimer's.
The topline data is expected to be disclosed in June 2023.
AL001 is a novel lithium-delivery system; it is a lithium-salicylate-L-proline engineered ionic cocrystal under development as an oral treatment for patients with dementia-related to mild, moderate, and severe cognitive impairment associated with Alzheimer's.
AL001 can potentially deliver the benefits of marketed lithium carbonate while mitigating or avoiding current toxicities associated with lithium.
"We strongly believe that AL001's patented ionic cocrystal technology could potentially provide clinicians with a major improvement over current lithium-based treatments and may constitute a means of treating over 40 million American suffering from Alzheimer's, bipolar disorder, MDD, and PTSD," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on March 22, 2023.
"We look forward to reporting topline data in June 2023 and further advancing clinical development of this promising potential therapeutic."
Having completed the clinical portion of the MAD study, the resulting pharmacokinetic and statistical data are undergoing evaluation of the safety and tolerability of AL001 under multiple-dose, steady‑state conditions.
This characterizes the maximum tolerated dose in healthy young and elderly subjects and subjects diagnosed with mild to moderate Alzheimer's.
Potentially safe and effective doses will be determined for deployment in planned subsequent Phase IIA clinical trials involving Alzheimer's, bipolar disorder, MDD, and PTSD subjects.
Lithium has been well-characterized for safety and is approved/marketed in multiple formulations for bipolar affective disorders.
AL001 lithium ascending dosing for the MAD cohorts tested incremental fractions of the usual lithium exposure for the treatment of bipolar affective disorder, with the target lithium dose for Alzheimer's treatment expected at a level that will not require therapeutic drug monitoring.
In each of the multiple healthy young/elderly and Alzheimer's cohorts, consisting of 6 active and 2 placebo patients each (as per randomization), multiple ascending doses were administered three times daily for 14 days under fasted conditions up to tolerability/safety limits that included the highest dose permitted per protocol.
As of March 22, 2023, there are no approved vaccines targeting Alzheimer's disease.
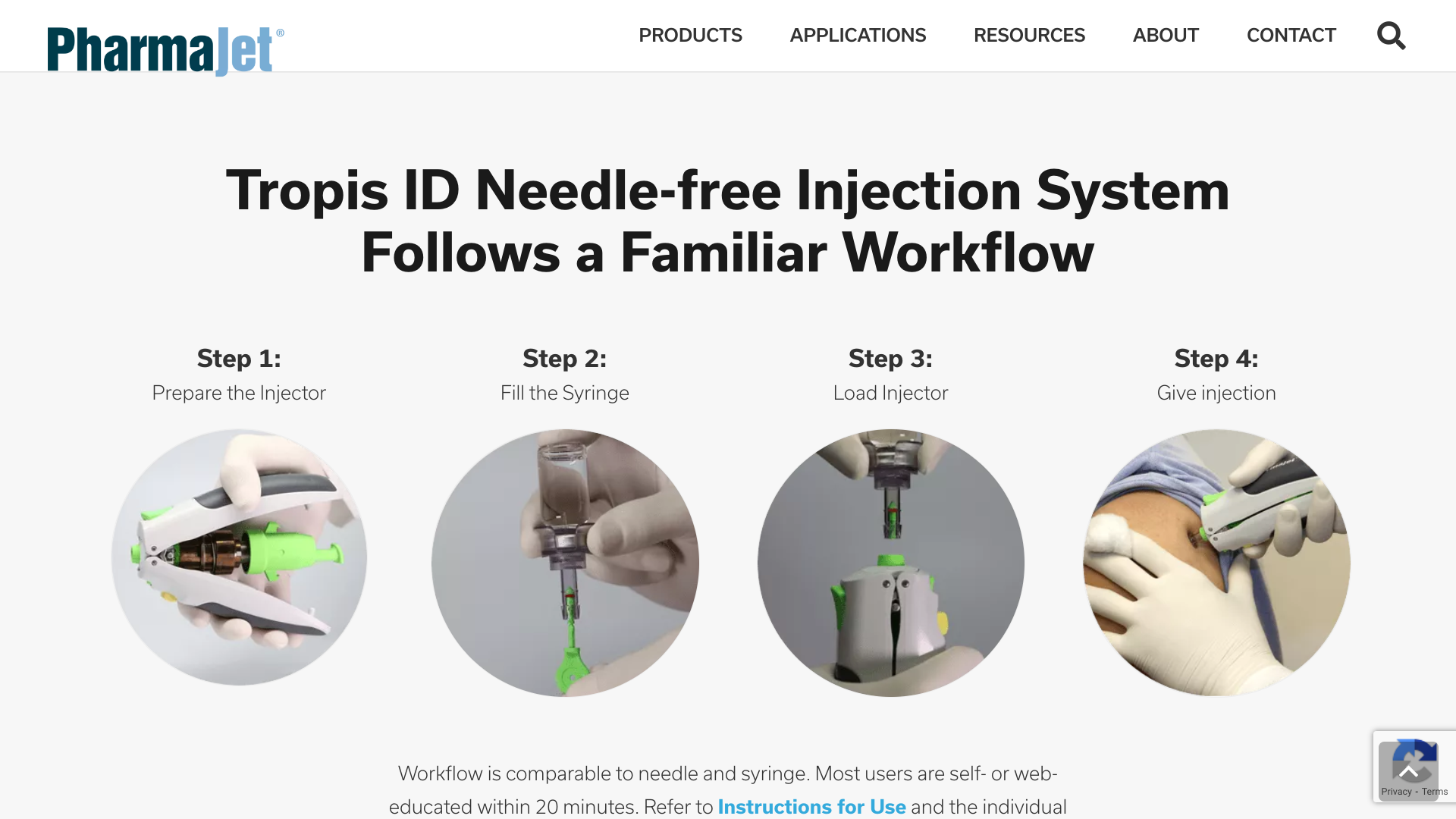
PharmaJet® today announced that its partner, Scancell, reported positive results from their Phase 1 COVIDITY clinical trial. The trial was conducted at the University of Cape Town Lung Institute in South Africa to assess the safety and immunogenicity of their COVID-19 DNA candidate vaccines, SCOV1 and SCOV2.
The results from the trial were highly encouraging, inducing neutralizing antibody and T cell responses with no safety concerns. Administration with PharmaJet's devices was well received by study participants.
This new set of human data adds to the growing evidence indicating that this modern needle-free administration technology is an increasingly viable option to enhance plasmid DNA vaccine immune response.
The vaccines were exclusively administered using the PharmaJet Tropis® and Stratis® needle-free precision delivery systems.
Professor Lindy Durrant, Chief Executive Officer, Scancell, commented in a press release on March 21, 2023, "We are encouraged by these results."
"The trial validates that AvidiMab®-modified immunotherapies boost immune responses and PharmaJet's Needle-free Injection Systems are effective in delivering our ImmunoBody®-generated drug candidate."
"Our plans are to include PharmaJet Needle-free precision delivery systems in future trials with our immuno-oncology projects."
PharmaJet Needle-free precision delivery Systems provide increased vaccine effectiveness, a preferred patient and caregiver experience, and a proven path to commercialization.
The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously.
The Tropis® System has CE Mark and WHO PQS certification for intradermal injections.
They are both commercially available for global immunization programs.
Note: This news article is not paid content.
Vaxxinity, Inc. today announced that the first subjects had been dosed in a randomized, double-blind, placebo-controlled Phase 1 clinical trial of VXX-401, an investigational vaccine designed to lower low-density lipoprotein (LDL) cholesterol, a known factor contributing to heart disease.
Heart disease remains the leading cause of death globally, claiming over 18 million deaths yearly.
VXX-401 is designed to induce robust, long-acting antibodies against PCSK9 to lower LDL cholesterol.
The multicenter Phase, 1 dose-escalation trial, aims to enroll 48 subjects aged 18 to 75 years with LDL cholesterol between 2.59 and 4.89 mmol/L.
The trial evaluates safety, tolerability, and immunogenicity (as measured by serum anti-PCSK9 antibody titers).
LDL cholesterol levels will measure the pharmacodynamics of the immune response, an established model of PCSK9 inhibition in hypercholesterolemia. This study was last updated on March 16, 2023.
VXX-401 was designed using Vaxxinity's proprietary synthetic peptide vaccine platform and is being developed to treat hypercholesterolemia.
The platform is designed to harness the immune system to convert the body into its own natural "drug factory," stimulating the production of antibodies.
"This is an exciting milestone for VXX-401 and Vaxxinity in our pursuit to vaccinate the world against heart disease with a preventative option that is convenient and accessible, addressing an unmet need to combat the leading global cause of death," said Mei Mei Hu, Chief Executive Officer of Vaxxinity, in a press release on March 20, 2023.
"PCSK9 antibody therapies are well-tolerated and effective, but huge, unmet patient need remains."
"In order to solve the problem of heart disease, the world needs a scalable, accessible technology that can reach the hundreds of millions, if not billions, of people at risk."
"With an LDL-lowering vaccine, we can offer an option that's cost-effective, safe, convenient, long-acting, and deployable."

Despite recent improvements in diagnostic tools, chikungunya outbreaks in Africa are probably underreported, stated a U.S. CDC Early Release Dispatch, Volume 29, Number 4—April 2023.
During 2019–2020, a large-scale chikungunya outbreak occurred in Djibouti City, the capital city of the Republic of Djibouti, located in the Horn of Africa.
Djibouti is a semi-arid country bordered by Eritrea, Somalia, and Ethiopia. In this region, the primary vector of the chikungunya virus (CHIKV) is the Aedes aegypti mosquito.
The chikungunya outbreak remained limited (attack rate 2.1%) but was followed by a dengue outbreak.
These researchers found clinical features helpful but insufficient to discriminate between chikungunya and dengue viruses.
However, CHIKV blood samples on blotting paper have been described as a field method for detecting arboviruses, routinely used in the French Armed Forces when deployed in Africa.
In this study, the researchers used blood samples on blotting paper to detect the emergence of CHIKV and monitor the course of the outbreaks.
Blotting paper provided a robust method for blood sampling and transport to a reference laboratory, making it possible to confirm 90% of the arboviral diagnoses.
We recommend blotting paper as a field tool to detect and monitor arboviral epidemics remotely, wrote these researchers.
Various countries, like Paraguay, are reporting chikungunya outbreaks in remote areas.
As of March 21, 2023, no chikungunya vaccines are authorized in Africa, Europe, or the U.S.
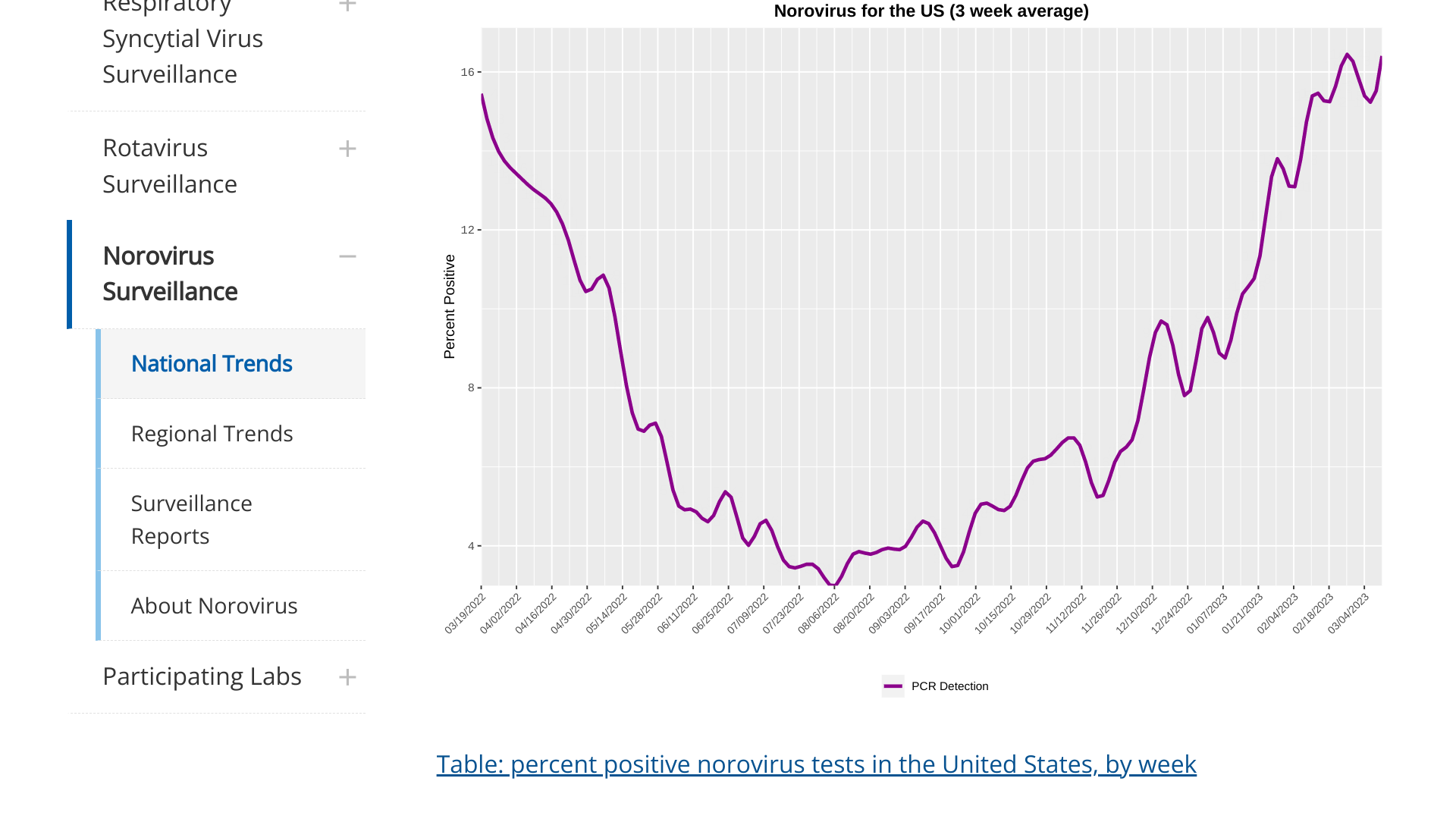
The National Center for Immunization and Respiratory Diseases recently reported data indicating cases of norovirus are spiking in the United States.
Norovirus is a very contagious virus that causes vomiting and diarrhea.
As of March 15, 2023, the 3-week moving average is reaching a new peak since the norovirus outbreak began in August 2022.
Each of the four regions has displayed similar trends, with the Northeast registering the steepest uptick.
In the U.S., cases of norovirus occur most frequently during late fall, winter, and early spring.
According to the U.S. Centers for Disease Control and Prevention (CDC), people can get norovirus illness many times in their life because there are many different types of noroviruses.
Infection with one type of norovirus may not protect you against other types.
The CDC says it is possible to develop immunity to specific types. But, it is not known exactly how long immunity lasts.
This may explain why so many people of all ages get infected during norovirus outbreaks. About 80% of children will experience a norovirus infection within one year of birth.
As of March 21, 2023, there are no approved norovirus vaccines in the U.S., Europe, or the U.K.
The peer review journal The Lancet Infectious Diseases published the results from a recent study examining prospective respiratory syncytial virus (RSV) surveillance data to assess the geotemporal prevalence of RSV A and B and functionally characterize the effect of the nirsevimab binding-site substitutions identified between 2015 and 2021.
Nirsevimab (Beyfortus®), an extended half-life monoclonal antibody (mAbs) to the RSV fusion protein, has been developed to protect infants for an entire RSV season.
This AstraZeneca and Sanofi-funded observational analysis concluded on March 17, 2023, nirsevimab binding site was highly conserved, and escape variants were rare and have not increased over time.
The U.S. Food and Drug Administration (FDA) initially approved an injectable mAbs therapy for children in 1998.
Beyfortus has been granted various regulatory approvals.
As of March 21, 2023, neither the FDA nor the European Medicines Agency approved an RSV vaccine candidate for children or older adults.
However, various authorizations are expected in 2023.

Regeneron Pharmaceuticals, Inc. and Sanofi today announced that the European Commission (EC) had approved Dupixent® in the European Union to treat severe atopic dermatitis in children aged six months to 5 years old who are candidates for systemic therapy.
With this approval on March 21, 2023, Dupixent is the first and only targeted medicine indicated to treat these children in Europe and the U.S.
Dupixent is a fully human monoclonal antibody injection administered under the skin at different injection sites.
Atopic dermatitis is a chronic type 2 inflammatory skin disease. Between 85% and 90% of patients first develop symptoms before five years of age, which can often continue through adulthood.
“Watching an infant or young child grapple with the debilitating and wide-reaching impacts of severe atopic dermatitis is heartbreaking,” said Korey Capozza, MPH, Founder and Executive Director of Global Parents for Eczema Research, in a press release.
“I’ve personally witnessed how this chronic skin disease can disrupt the lives of entire families when left uncontrolled. However, intervening with effective treatments during infancy and early childhood can help manage the challenging impact this disease has on children and their families during such formative years.”
Severe atopic dermatitis may also significantly impact the quality of life of young children and their caregivers. Treatment options in this age group are primarily topical corticosteroids, which can be associated with safety risks and may impair growth when used long-term.
The approval is based on data from a Phase 3 trial evaluating Dupixent every four weeks (200 mg or 300 mg based on body weight) plus low-potency primarily topical corticosteroids (TCS) or TCS alone (placebo) in 162 children aged six months to 5 years with moderate-to-severe atopic dermatitis.
At 16 weeks, Dupixent improved skin clearance and reduced overall disease severity and itch compared to placebo in the overall enrolled population. However, in a subset of those with severe atopic dermatitis, patients randomized to Dupixent (n=63) experienced the following compared to placebo (n=62) at 16 weeks:
- In addition, 46% of patients achieved 75% or greater improvement in overall disease severity compared to 7% treated with placebo, a co-primary endpoint.
- 14% of patients achieved clear or almost clear skin compared to 2% treated with placebo, a co-primary endpoint.
- 55% average reduction in overall disease severity from baseline compared to 10% with placebo.
- 42% average reduction in itch from baseline compared to a 1% increase with placebo.
Dupixent also improved sleep quality, skin pain, and health-related quality of life compared to placebo in both the overall and severe populations. In addition, long-term efficacy data showed the clinical benefit at 16 weeks was sustained through 52 weeks.
The most common side effects across indications include injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes, and eosinophilia.
Dupixent is currently approved for one or more indications in more than 60 countries, including Europe, the U.S., and Japan. More than 600,000 patients are being treated with Dupixent globally.



