Search API
Public Health France recently announced an investigation of Mpox cases clustered in the Centre-Val de Loire region, located in the Paris Basin and bisected by the Loire River.
As of March 23, 2023, about 5,000 cases of Mpox had been recorded in France since May 2022.
After the peak of contamination reached the end during June/July, the number of cases fell sharply.
Between January 2023 and March 23, 2023, 17 confirmed male cases were reported in the Centre-Val de Loire region, including 14 since March 1, 2023.
Six of the 17 cases had received no smallpox vaccination.
Unfortunately, Mpox infections can occur in people who have received a complete 2-dose vaccination.
The investigation showed that all these cases concerned men who have sex with men, several of whom reported having had several partners without always being able to identify them.
In addition, no parties or events common to the cases were identified.
The clinical characteristics of these cases remain similar to those previously observed; no case required hospitalization.
In addition, Mpox outbreak news is posted at MpoxToday.

Following assessing the primary endpoints of the Phase II/III study, PharmaJet partner Gennova Biopharmaceuticals Limited has submitted data for its mRNA-based Omicron-specific Covid-19 booster shot for Emergency Use Authorization to the office of the Drug Controller General of India.
This is the first booster in India explicitly targeting the SARS-CoV-2 coronavirus Omicron variant.
GEMCOVAC-OM is a lyophilized vaccine, stable at 2-8 °C, which means it can be distributed through the existing refrigeration supply chain Pan-India and in low- and middle-income countries.
The vaccine, GEMCOVAC-OM, will be delivered exclusively with the PharmaJet Tropis Precision Delivery System.
"Our partnership with Gennova Pharma and their Omicron booster clinical program confirms the value of choosing our widely validated and rapidly scalable Precision Delivery Systems to improve the effectiveness of DNA and mRNA vaccines," said Chris Cappello, President, and CEO, PharmaJet, in a press release on April 3, 2023.
"The Tropis System is already commercially available in India, and we are well-prepared to rapidly support demand for the GEMCOVAC-OM Omicron booster."
"In addition, we look forward to continuing our partnership with Gennova with additional novel vaccines."
For more information, visit Pharmajet's website.

Alzamend Neuro, Inc. today announced the initiation of a phase I/IIA clinical trial for its immunotherapy vaccine (ALZN002) to treat mild to moderate dementia of Alzheimer's Disease.
ALZN002 is a proprietary "active" immunotherapy product, which means each patient's immune system produces it.
This trial aims to assess the safety, tolerability, and efficacy of multiple ascending doses of ALZN002 compared with that of placebo in 20-30 subjects with mild to moderate morbidity.
"Alzamend's motto is 'Making Alzheimer's just a memory.' There remains a need to develop new therapies that alter the progression of Alzheimer's and prevent, reverse, or slow neurodegeneration and cognitive decline."
"Today, we are on the threshold of importantly advancing the art and science of anti-beta amyloid therapy by treating each Alzheimer's patient's immune system," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on April 3, 2023.
"Intermittent use of our immunotherapeutic vaccine (ALZN002) may be expected to limit the number of infusions needed, may reduce the potential for adverse reactions, and provide more substantive cognitive and functional outcomes to the millions of Americans afflicted with this devastating disease."
The primary goal of this clinical trial is to determine an appropriate dose of ALZN002 for treating patients with Alzheimer's in a more extensive Phase IIB efficacy and safety clinical trial, which Alzamend expects to initiate within three months of receiving data from the initial trial.
Other Alzheimer's disease vaccine news is posted at this link.

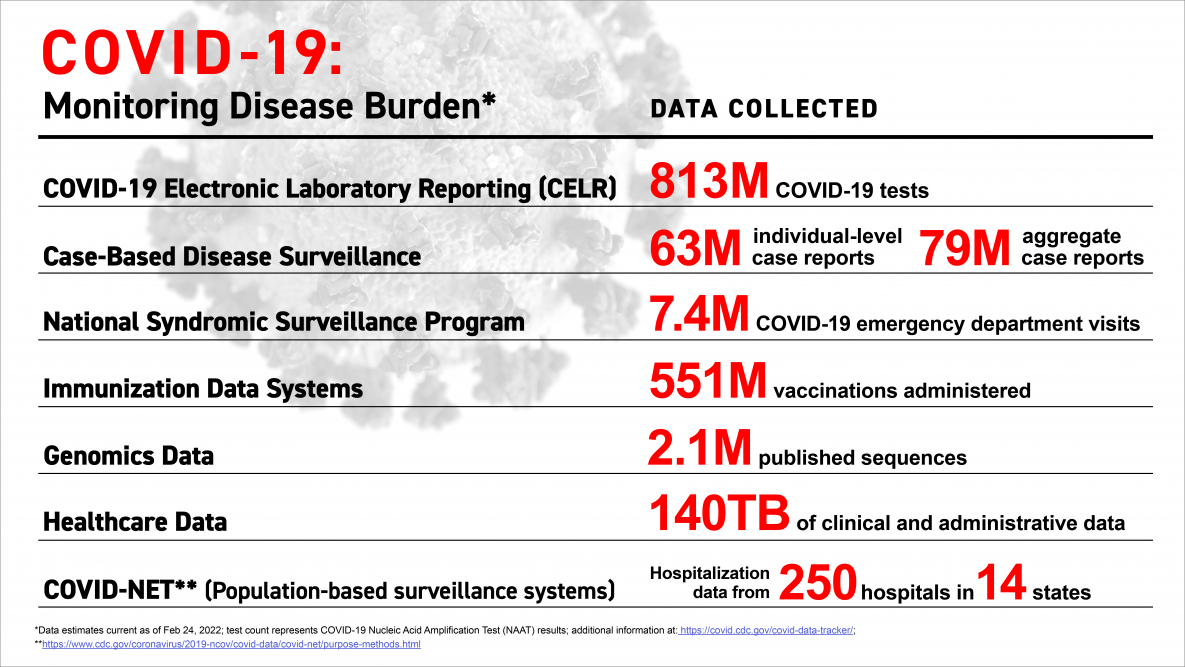
The COVID-19 pandemic fundamentally changed expectations for public health, especially the speed at which credible health information is delivered, wrote the U.S. Centers for Disease Control and Prevention (CDC).
Throughout the pandemic, the CDC has been improving the timeliness, completeness, and quality of critical data for the response.
Through the Data Modernization Initiative, the CDC empowered 'data to move faster than a disease.'
Updated on March 31, 2023, a series of Weekly Review issues coinciding with the end of the COVID-19 public health emergency (May 2023) and what it means for CDC and the data reported.
The first issue was published on February 24, 2023, and the final two issues will publish on April 14 and May 12, 2023.
To view current and historical maps of COVID-19 vaccination by demographics in the U.S. as of April 2, 2023, please visit this webpage: Maps of COVID-19 Vaccinations by Age and Sex over Time.
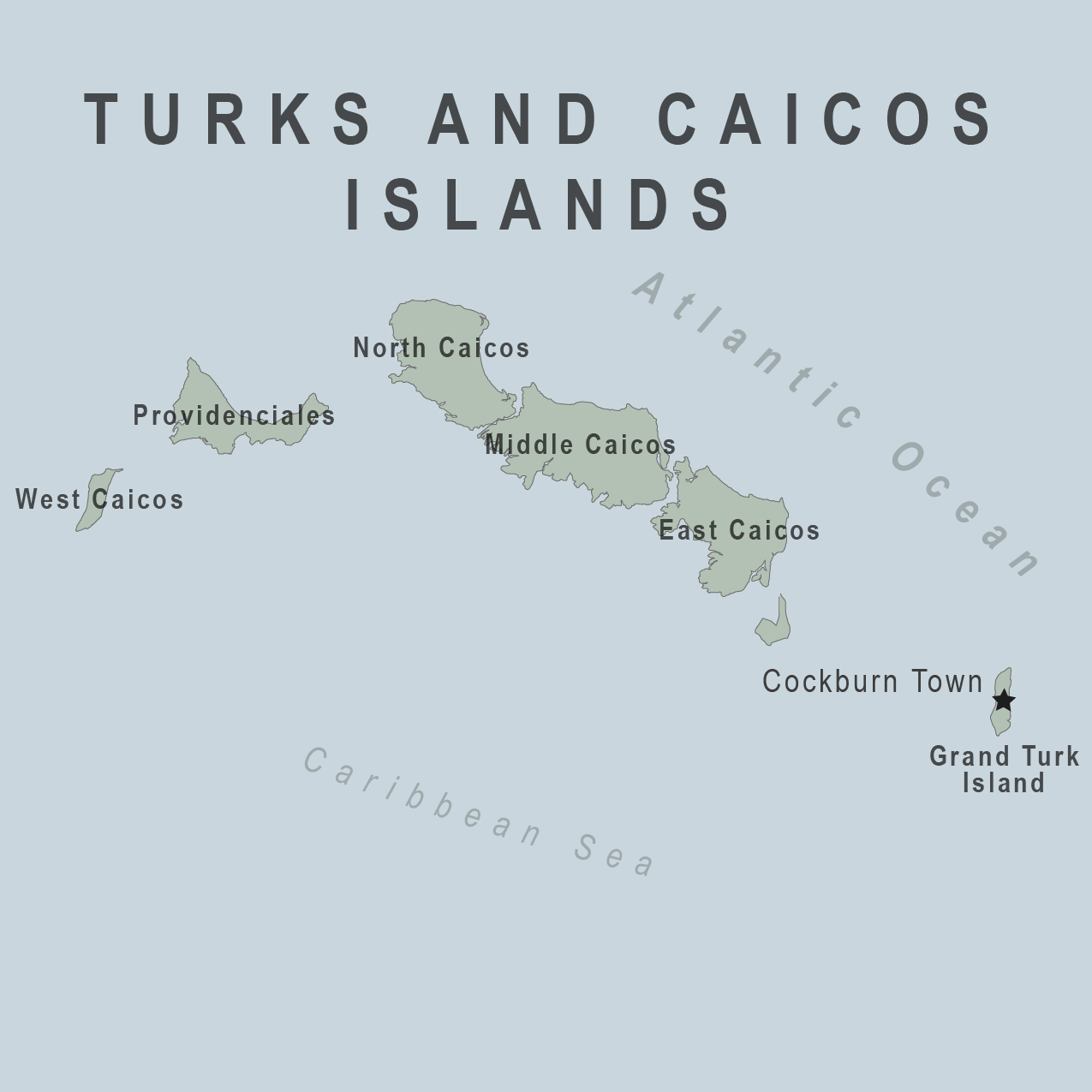
There are new entry requirements when visiting the Turks and Caicos Islands. The Ministry of Health and Human Services recently announced the COVID-19-related requirements would expire.
This means proof of COVID-19 vaccination will no longer be required for visitors to the Turks and Caicos Islands as of April 1, 2023.
However, persons are strongly encouraged to take general precautions to protect themselves and limit the transmission of the SARS-CoV-2 coronavirus and other respiratory illnesses in circulation.
And eligible persons are strongly encouraged to consider taking COVID-19 vaccine/booster and the flu shot.
These vaccines are available at no cost at all Primary Health Care Clinics. For further information, please visit the Ministry of Health’s website https://www.gov.tc/moh/coronavirus/.
Separately, the U.S. CDC suggests other travel vaccinations when visiting Turks and Caicos in 2023.
Furthermore, there is a risk of dengue in this country, and visitors should avoid mosquito bites, particularly between dawn and dusk.
A British Overseas Territory located southeast of the Bahamas, Turks and Caicos is an archipelago of 40 islands in the Atlantic Ocean. Before the recent pandemic, about 1.5 million people visited the islands in 2019.
Lucira Health, Inc. recently announced the nationwide launch of its Lucira COVID-19 & Flu Home Test in the United States.
The COVID-19 & Flu Home Test is the first and only combination COVID-19 & Flu test granted emergency use authorization by the U.S. Food and Drug Administration for use at home and other non-laboratory sites.
The Lucira COVID-19 & Flu Home Test is a molecular test, not an antigen test, that demonstrated similar performance for COVID-19 and Influenza compared to highly sensitive lab-based PCR tests in clinical trials.
The easy-to-use, all-in-one combination test delivers results in 30 minutes or less from one shallow nasal swab and as of March 28, 2023, can be purchased in the U.S. for $34.99 at www.lucirahealth.com/flu.

The Global Polio Eradication Initiative (GPEI) recently announced it had been two years since the novel oral polio vaccine type 2 (nOPV2) became available.
Approximately 590 million doses of nOPV2 have been administered across 28 countries under its WHO Emergency Use Listing (EUL) to date.
An additional 13 countries have met the EUL requirements for nOPV2 use in the event of a polio outbreak.
As of April 1, 2023, the nOPV2 vaccine is unavailable in the U.S.
GPEI’s nOPV Working Group leaders, Simona Zipursky (World Health Organization) and Ananda S. Bandyopadhyay (Bill & Melinda Gates Foundation), were interviewed on March 29, 2023, on the journey and the vaccine’s performance so far.
The unedited interview is posted at this link.

A recent study published in the peer-reviewed journal Science Advances indicates that the protease inhibitor Paxlovid could soon become less effective in treating COVID-19 infections.
This new research shows that simple single amino acid changes in SARS-CoV-2 coronavirus main protease could severely undermine the efficacy of antiviral drugs.
To lower the risk of resistance, the researchers say protease inhibitors must be carefully designed to avoid simple resistance mutations.
Announced on March 29, 2023, and conducted by the Midwest Antiviral Drug Discovery Center in Minnesota, this study shows that drug-resistant variants have appeared multiple times independently in different parts of the world, with regional clusters providing evidence for person-to-person transmission.
Further research will likely develop additional next-generation protease inhibitors with different resistance profiles and drugs targeting other viral processes, such as replication or cell entry.
A multi-drug approach—like existing therapies for HIV and Hepatitis C virus — could further help to protect against resistance and cure SARS-CoV-2-infected individuals, wrote these researchers.

The UK Health Security Agency Week #13 reported COVID-19 activity decreased across most indicators compared with the previous week.
As of March 30, 2023, through Respiratory Datamart, SARS-CoV-2 positivity decreased to 10% compared with 10.9% in the previous week.
Overall, COVID-19 hospitalizations decreased slightly in week #12 and were highest in the 85 and over age group.
Furthermore, the COVID-19 Autumn booster vaccination campaign commenced in September 2022.
By the end of week #10, about 65% of all people aged over 50 and living in England had been vaccinated with an Autumn booster dose.

