Search API
The World Health Organization (WHO) published a Weekly Epidemiological Update Edition #137 regarding the COVID-19 pandemic on April 5, 2023.
The WHO disclosed a decrease of 28% in new COVID-19 cases and 30% in related fatalities, respectively, compared to the previous 28 days (February 6 to March 5, 2023).
Despite this overall downward trend, it is essential to note that 74 (31%) countries have reported increases in new cases of 20% or greater during the last 28 days compared to the previous 28-day period.
As of April 2, 2023, over 762 million confirmed cases and over 6.8 million deaths have been reported globally.
Furthermore, the WHO continues Listing numerous COVID-19 vaccines available in various countries.

BioSpace reported today Vivek Shinde, vice president of clinical development and lead for older adult influenza & RSV vaccines at Novavax Inc., delivered an update on the Company's COVID-influenza combination (CIC) candidate at the World Vaccine Congress 2023.
The Company presented CIC data and poster presentations on its COVID-19 prototype vaccine during this conference in Washington, DC.
Shinde confirmed that CIC formulations were immunogenic and induced strong functional antibody and CD4+ T cell responses against SARS-CoV-2 and multiple influenza strains on April 5, 2023.
"I think a combination vaccine is only going to hopefully boost compliance in those who intend to get both vaccines," he informed Heather McKenzie at BioSpace on April 7, 2023.
Novavax intends to use these data to inform an ongoing Phase II dose confirmation study. Results from this study are expected later in 2023.
Novavax's COVID-19 vaccine (Nuvaxovid™, CovoVax™, NVX-CoV2373) protein-based vaccine has received various authorizations from more than 43 countries.

Researchers at the Oak Crest Institute of Science have been exploring new ways to prevent the spread of HIV-1 among young women in low and middle-income countries.
Some questions have been answered in a new study, and the answers may be surprising.
In a recent study published by the journal Nature on March 21, 2023, using humanized mice (mice that have received implants of human cells and tissues so that they can be infected with HIV-1), researchers tested the vaginal HIV-1 prevention efficacy of single and combination antiviral compounds applied locally.
They used a mathematical model to empirically study the effects of administering different doses of antiviral drugs.
The results were unexpected: when tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) – both drugs that share the same mechanism of inhibiting HIV-1 reverse transcriptase, thereby preventing viral replication in immune cells – were combined with a third drug that used a different mode of action against HIV-1, a strong antagonistic effect was observed.
This means that the drugs were less effective when used together than they were when used separately.
'Our approach provides a translational template for the preclinical, rational, and systematic evaluation of drug combinations for the prevention of HIV-1 and other viral diseases,' wrote these researchers.
As of April 7, 2023, there are no approved HIV vaccines available.

The Wyoming State Veterinary Laboratory (WSVL) recently diagnosed the highly pathogenic avian influenza (HPAI) virus in a barn cat near Thermopolis, WY. This is the first report of HPAI in a domestic cat in Wyoming, and it likely became infected from ingesting meat from wild waterfowl.
'We know that wild birds, particularly waterfowl, have been affected in large numbers by HPAI, but in recent months we have detected the virus in wild carnivorous mammals, including mountain lions and a red fox,' wrote WSVL in April 2023.
As the HPAI outbreak continues, please ensure you use gloves and masks when handling sick or dead mammals and birds. And report any sick wildlife through the Wyoming Game and Fish Department's Wildlife Health Laboratory online reporting tool at this link.
Additionally, various mammals infected with bird flu have been reported in Canada, Mexico, and South America in 2023.
And about eleven humans have recently been infected with this version of bird flu.
As of April 7, 2023, there are U.S. Food and Drug Administration-approved avian influenza vaccines, with several vaccine candidates conducting studies. Furthermore, annual flu shots are not effective against bird flu viruses.

The Centers for Disease Control and Prevention (CDC) recently issued Health Alert Network Health Advisory CDCHAN-00489, informing clinicians and public health departments about Marburg virus disease (MVD) outbreaks in Equatorial Guinea and one in Tanzania.
As of April 7, 2023, there is no evidence to suggest that these outbreaks are related, as most experts agree that these represent two independent animal-to-human spillover events.
To date, no confirmed cases of MVD related to these outbreaks have been reported in the U.S. or other countries outside Equatorial Guinea and Tanzania.
This CDC Health Advisory aims to increase awareness of the risk of imported cases in the U.S.
It also summarizes CDC’s recommendations for case identification, testing, and clinical laboratory biosafety considerations in the U.S.
Furthermore, the CDC confirmed no vaccines or treatments are authorized specifically for MVD.
ADvantage Therapeutics, Inc. today announced that the U.K.'s Medicines and Healthcare Products Regulatory Agency granted the Company's lead compound AD04™ an Innovation Passport for treating Alzheimer's disease (AD) under the Innovative Licensing and Access Pathway (ILAP).
ILAP was established in 2021 to reduce the time to market innovative medicines in the U.K.
A Phase 2b trial for AD04™ in the ILAP application will be a randomized, placebo-controlled, double-blind study to confirm proof of concept and establish the safety and efficacy of AD04™ in mild AD patients.
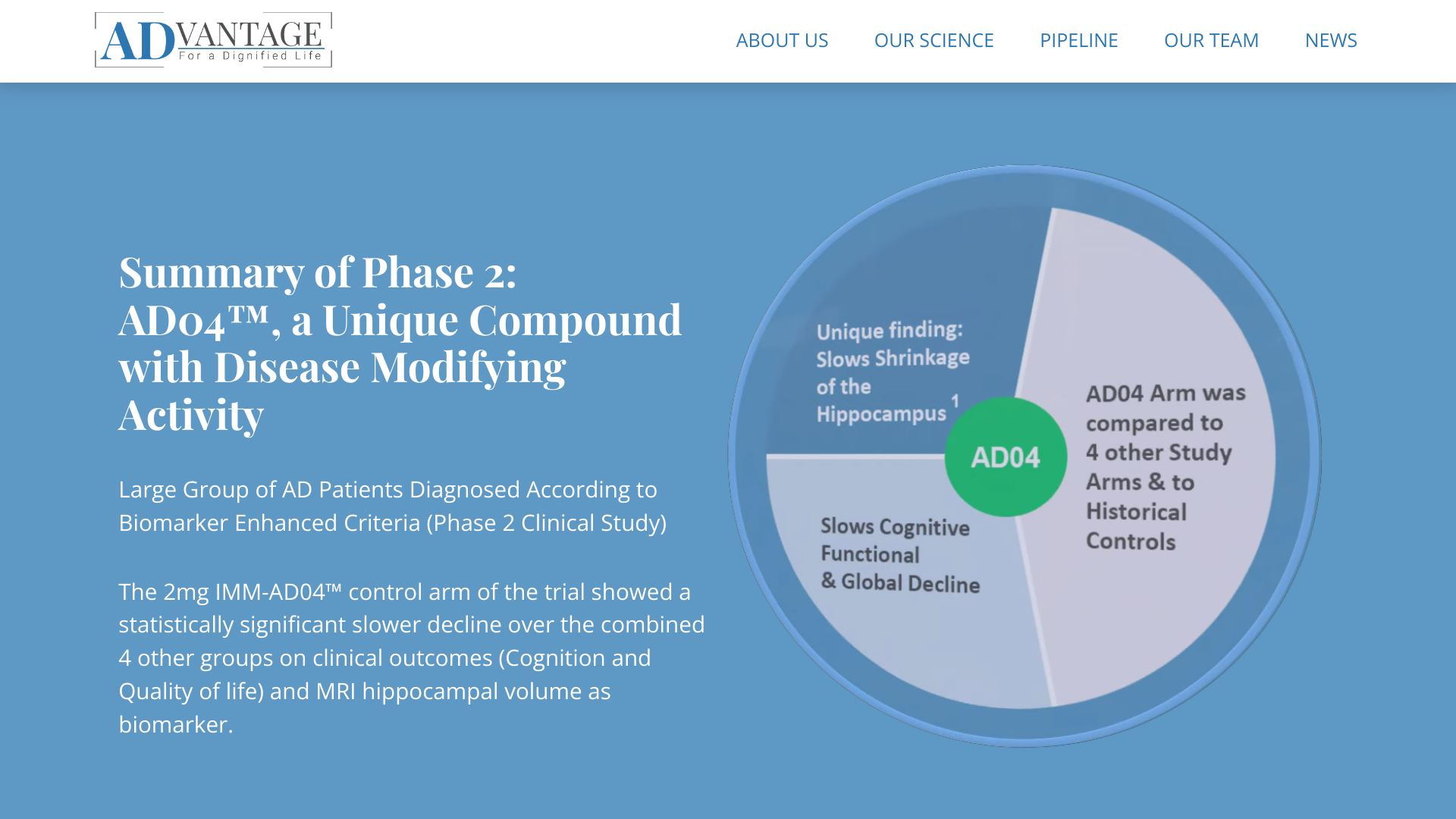
A 2mg dose of AD04™ served as a control in a prior study of another compound, where it demonstrated a statistically significantly slower decline in outcomes (cognition, function, and quality of life) than other arms of that trial.
In addition, the AD04™ control group showed a slower decline in MRI-measured hippocampal volume as a biomarker of AD progression.
To date, preclinical studies have shown that AD04™ decreased the number of inflammatory microglial cells in the hippocampus of mouse models. Inflammation and hyperproliferation of microglial cells are pathological immunological events in the brain of Alzheimer's patients.
Dr. Rudolph Tanzi, who serves as Chair of the Scientific Advisory Board at ADvantage, stated in a press release on April 5, 2023, "The democratization of AD treatments that are safe, effective, and affordable for all are of utmost importance."
"We hope this AD04 trial with ILAP designation will bring us one step closer," he concluded.
ADvantage is located in Miami, Florida, and is developing potentially paradigm-changing therapies to treat AD in its labs at the Vienna BioCenter.
Additional AD vaccine and treatment news are posted at Precision Vaccinations.
Updated on April 11, 2023, with related links.
Shionogi & Co., Ltd. today announced that two late-breaking poster presentations featuring results from clinical trials on its novel COVID-19 oral antiviral ensitrelvir would be published as posters at the 33rd European Congress of Clinical Microbiology & Infectious Diseases.
Ensitrelvir, known as Xocova® 125 mg tablet in Japan, received emergency regulatory approval from the Ministry of Health, Labour and Welfare (MHLW) to treat SARS-CoV-2 infection.
Recently, ensitrelvir was granted Fast Track designation by the U.S. Food and Drug Administration (FDA).
The first late-breaking poster presentation included a post-hoc analysis of the Phase 3 part showing that viral rebound and symptom recurrence were infrequently seen up to 21 days after treatment with ensitrelvir.
Viral RNA rebound by PCR testing was observed in 7.8% of the ensitrelvir 125 mg group (n=590) and 4.7% in the placebo group (n=574). Symptom recurrence was rare and was not associated with viral RNA rebound.
Although RNA rebound was observed in a few patients, there was only one (1/310) low-level viral titer positive in follow-up, suggesting no concerns for infectivity or transmission.
A second late-breaking poster presentation included new results from the study (Phase 2b/3 part) of patients who tested positive for SARS-CoV-2 but were either asymptomatic or had only mild symptoms at the time of randomization.
These results were based on 572 patients who were followed up for ten days after randomization. Ensitrelvir 125 mg showed a significant reduction from baseline viral RNA on Day 4, a reduction of 1.12 log10 copies/mL versus placebo (p<0.0001).
The time to the first negative SARS-CoV-2 culture was significantly shorter with ensitrelvir 125 mg compared to placebo (a median time of 38.3 hours versus 66.7 hours, p<0.0001, respectively).
Although these results were exploratory, the reduction in viral RNA and faster time to a negative viral culture may be predicted to reduce the period of infectivity, which may have implications for reducing the risk of transmission.
In a subset of 70 asymptomatic patients, ensitrelvir 125 mg (n=23) showed a numerical reduction in the proportion of patients developing symptoms.
In the 502 patients presenting with mild symptoms treated with ensitrelvir 125 mg (n=171), a numerical reduction in the proportion reporting a worsening of symptoms compared with placebo was observed.
Ensitrelvir was well tolerated, and no new safety concerns were identified.
A separate Phase 3 study of ensitrelvir (SCORPIO-HR) is underway across Asia, Africa, North America, and Europe in non-hospitalized adults who have tested positive for SARS-CoV-2. It includes those with and without risk factors for severe disease and regardless of vaccination status. Shionogi also plans to initiate a post-exposure prevention global Phase 3 study, SCORPIO-PEP.
“We are encouraged by these new data regarding the potential reduction of transmission among asymptomatic patients and patients with mild COVID-19 symptoms,” said Isao Teshirogi, Ph.D., in a press release on April 5, 2023.
“We are continuing to evaluate ensitrelvir in multiple patient populations through our robust global clinical program and look forward to continued scientific exchange on this important compound.”
Ensitrelvir remains an investigational drug outside of Japan and has not been approved outside of Japan.
Other COVID-19 antiviral news is posted at Coronavirus Today.

The Canadian Food Inspection Agency (CFIA) recently confirmed a dog in Ontario had contracted avian influenza (bird flu) and died.
The CFIA reported on April 1, 2023; the dog was infected with bird flu after chewing on a wild goose.
“Based on the current evidence in Canada, the risk to the general public remains low, and current scientific evidence suggests that the risk of a human contracting avian influenza from a domestic pet is minor,” the CFIA stated.
Canada has previously reported bird flu infections have led to the demise of bears, foxes, and skunks.
Other bird flu news is posted at PrecisionVaccinations.com/BirdFlu.

The United Arab Emirates Ministry of Health and Prevention (MoHAP) today announced it urges the public to be aware of the Marburg virus causing hemorrhagic fever.
And to avoid traveling to Tanzania and Equatorial Guinea unless necessary due to the recent spread of the Marburg virus.
The MoHAP said in a statement on April 4, 2023, if travel is unavoidable, necessary precautions should be taken to avoid exposure to the disease, such as avoiding close contact with patients, touching contaminated surfaces, and refraining from visiting caves and mines.
This announcement comes after several Arab countries have already advised their citizens to postpone traveling to those two countries due to the same concern.
Both the U.S. Department of State and Centers for Disease Control and Prevention have issued travel alerts related to these Marburg outbreaks.
As of April 5, 2023, no Marburg preventive vaccine is authorized in the U.S.

