Search API
Gritstone bio, Inc. today presented 6-month follow-up data from its ongoing Phase 1 CORAL-CEPI and CORAL-BOOST studies, which are evaluating the company's self-amplifying mRNA (samRNA) vaccine candidates against SARS-CoV-2, at the 33rd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
"The results shared today demonstrate that regardless of whether and/or how the immune system was previously exposed to SARS-CoV-2, our samRNA vaccine candidates are driving robust antibody titers that are sustained for at least six months in healthy adults," said Karin Jooss, EVP and Head of R&D at Gritstone bio, in a press release on April 17, 2023.
"Current vaccines against COVID-19 have demonstrated susceptibility to loss of immunity over time, posing a greater burden on individuals and our health systems."
"Developing a next-generation vaccine that drives more durable and broad neutralizing antibodies against variants of concern not included in the vaccine – both of which are demonstrated in these results - could serve as a key factor in delivering long-term, variant-proof immune protection."
"The results shared today further support our hypothesis that samRNA, unique from mRNA due to several distinct characteristics, could serve as a widely applicable next-generation vaccine platform technology against SARS-CoV-2 and beyond."
Gritstone's CORAL program applies an infectious disease approach, which aims to drive B cell and T cell immunity using samRNA against SARS-CoV-2.
The program serves as proof of concept for applying Gritstone's platform against coronaviruses and other infectious diseases. The Bill & Melinda Gates Foundation, the U.S. NIAID, and the Coalition for Epidemic Preparedness Innovations support it.
SAB Biotherapeutics announced today that the U.S. Food and Drug Administration (FDA) had granted Breakthrough Therapy Designation to SAB-176, an investigational therapeutic, for post-exposure prophylaxis for Type A and Type B influenza illness in high-risk patients, including those who have antiviral resistant strains.
SAB-176 is being developed for several influenza indications.
The FDA's Breakthrough Therapy designation confirms that the multi-epitope targeting modality of SAB-176 has a clear differentiation vs. monoclonal antibodies (mAbs) that bind to a single epitope.
And SAB's treatment can sustain its efficacy over viral mutations and prevent or reduce the risk of emerging treatment-resistant influenza strains.
Virus evolution driven by vaccines or treatments is a serious challenge, and the use of therapeutics can create "escape mutants" or versions of a virus that have changed to escape pressure on virus survival driven by antiviral treatment, whether it is a small molecule or mAbs modality, wrote the company.
SAB recently announced that the FDA had granted Fast Track designation to SAB-176.
The company had also received FDA guidance and regulatory alignment on advancing SAB-176 into the next development phase by initiating a Phase 2b clinical trial.
"Influenza continues to pose considerable health concerns in the U.S. and globally. This Breakthrough Therapy designation signifies an important step forward in our fight against this disease," said Eddie Sullivan, Ph.D., co-founder, President & CEO of SAB Biotherapeutics, in a press release on April 18, 2023.
"We are proud that based on generated preclinical and clinical evidence, SAB-176 has received both Breakthrough and Fast Track designations, a combination rarely seen."
The FDA's Breakthrough Therapy designation process is designed to expedite the development and reviewing a medicine intended to treat a serious or life-threatening condition. Preliminary clinical evidence indicates that the drug may substantially improve over current therapies on a clinically significant endpoint(s).
Products that qualify for Breakthrough Therapy designation receive more benefits than Fast Track products.
Precision Vaccinations post influenza vaccines news for April 2023.

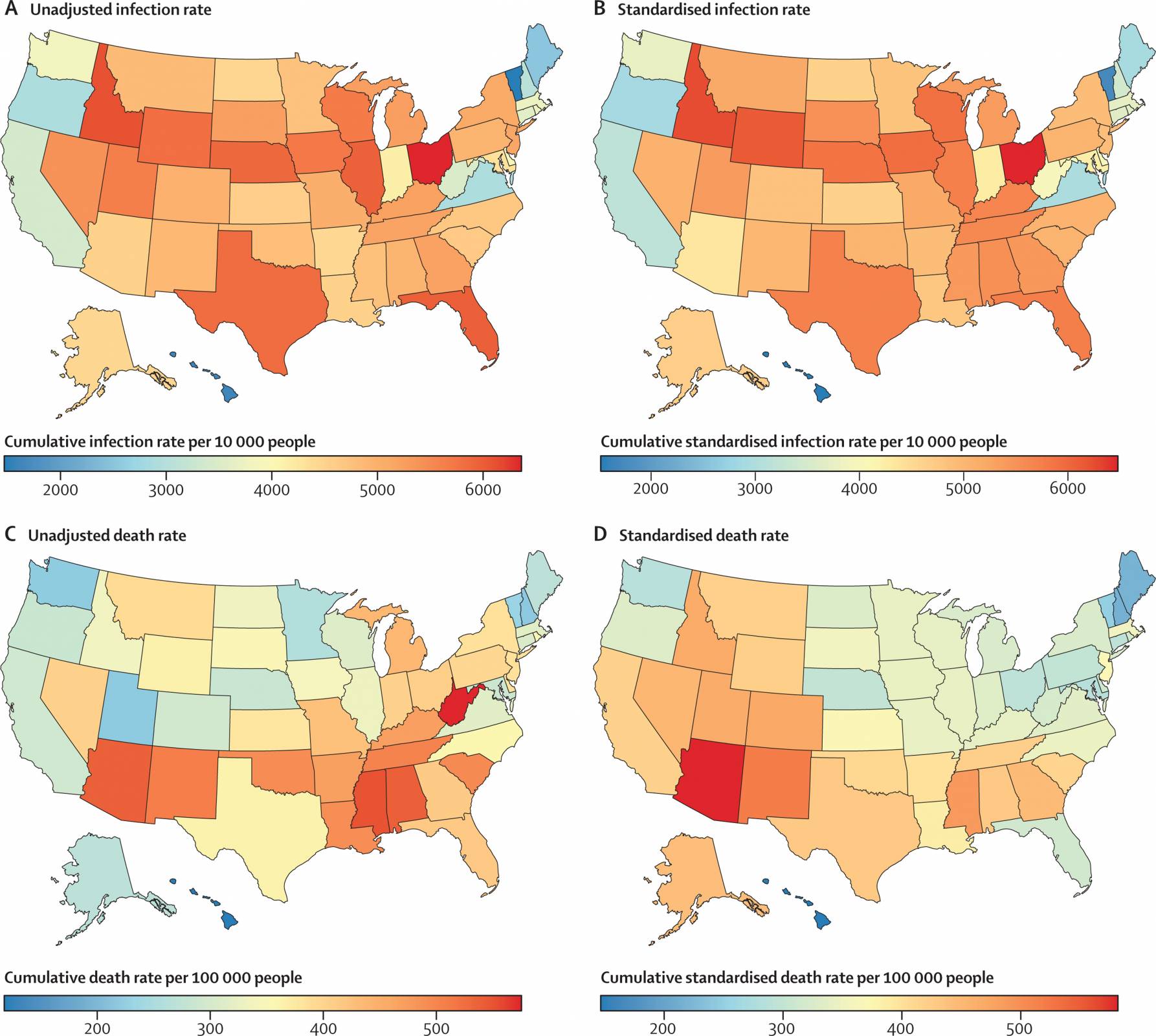
The Lancet recently published results from an analysis that assessed COVID-19 pandemic policies and behaviors in the U.S.
These researchers revealed where a person lived indicated COVID-19 risk.
Published on March 23, 2023, this analysis found nearly four-fold differences that existed across states in COVID-19 death rates, even when standardized for factors such as age and comorbidities, suggesting that lower death rates were achievable.
The states with the lowest standardized COVID-19 death rates were Hawaii, New Hampshire, Maine, Vermont, and Maryland, which are not confined to a single geographical region.
And the states and territories with the highest standardized cumulative death rates were Arizona (581 per 100 000 [509–672]), Washington, DC, New Mexico, Mississippi, and Colorado.
In summary, these researchers stated that the policy mandates and protective behaviors adopted during this pandemic effectively reduced SARS-CoV-2 infections.
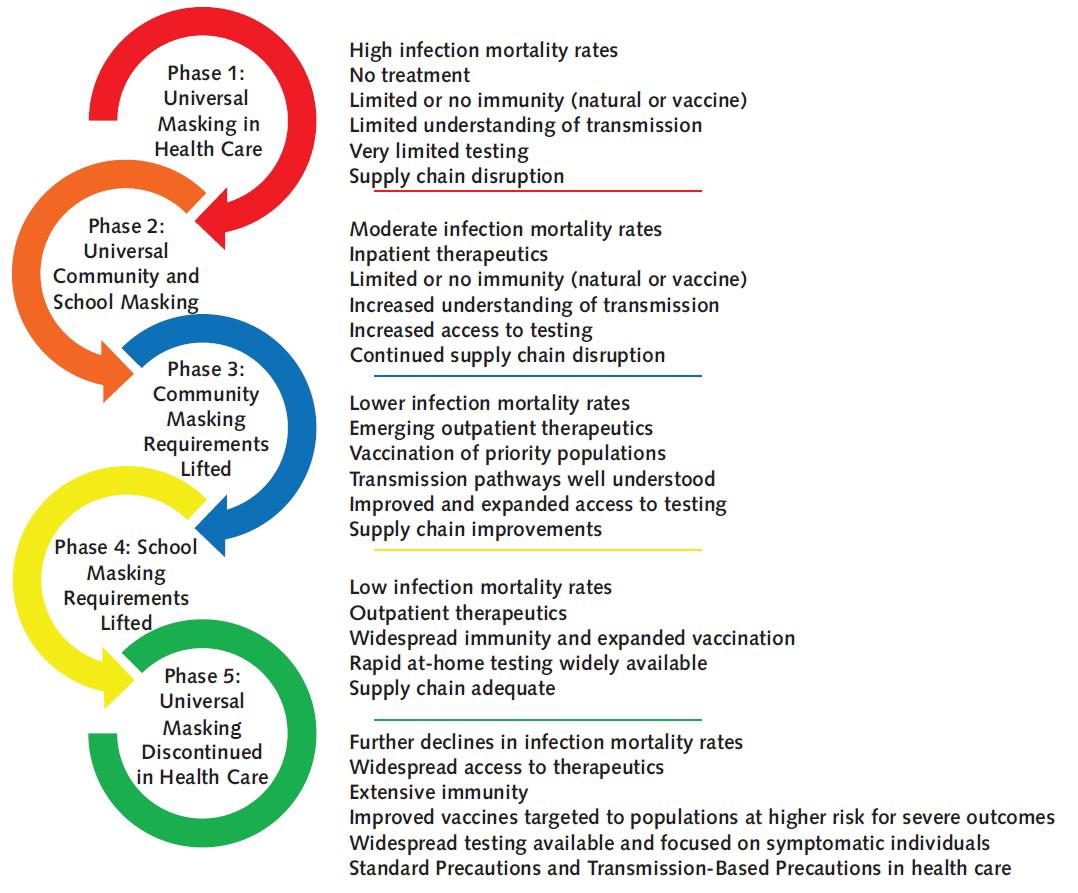
The Annals of Internal Medicine today published an opinion article that confirmed during the COVID-19 pandemic, the expanded use of facemasks as part of “universal masking” for healthcare personnel (HCP), patients, and visitors in healthcare settings was implemented to reduce the risk for morbidity and mortality associated with the spread of a novel virulent pathogen.
However, the context and conditions of the pandemic have changed dramatically, and evidence-based public health policy should also adapt in response.
The time has come to manage SARS-CoV-2 as we generally manage other endemic respiratory viruses in healthcare settings through correct and consistent application of Standard Precautions and Transmission-Based Precautions.
Moving away from universal masking policies should be accompanied by reconsidering other pandemic-era strategies, such as asymptomatic testing and resource-intensive contact tracing.
In conclusion, this article published on April 18, 2023, stated .... Interactions between humans and pathogens are inherently dynamic.
Therefore, they are constantly evolving, and we have achieved significant advancements in preventing and managing SARS-CoV-2 since the pathogen was initially identified in 2019.
In recognition of these achievements, the time has come to deimplement policies inappropriate for an endemic pathogen when the expected benefits of such policies are low.
Universal masking in health care is a policy whose time has come and gone ... for now.
Face mask research is posted by Coronavirus Today.
Prof Mojisola Christianah Adeyeye, Director-General of the Federal Republic of Nogeria's National Agency for Food and Drug Administration And Control (NAFDAC), today announced it granted registration approval for the R21/Matrix-M™ vaccine.
The NAFDAC approval on April 17, 2023, is essential since the WHO African Region continues to carry a disproportionately high share of the global malaria burden.
For example, in 2021, the Region was home to about 95% of all malaria cases and 96% of deaths.
And malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
Furthermore, the prevalence of malaria parasitemia in Nigerian children under five is about 23%.
The R21 was created by the University of Oxford Jenner Institute in England, is manufactured by Serum Institute of India Pvt. Ltd., and includes Novavax AB proprietary saponin-based Matrix-M adjuvant.
In addition to malaria, the U.S. CDC has issued various Travel Advisories regarding disease outbreaks in Nigeria.
Vaccine-preventable diseases such as yellow fever, measles, and polio are health risks when visiting Nigeria in 2023.
The Nigerian National Agency for Food and Drug Administration and Control (NAFDAC) today announced its approval for the R21/Matrix-M™ Malaria Vaccine manufactured by India's Serum Institute of India Pvt. Ltd.
The Marketing Authorization Holder is Fidson Healthcare Ltd.
During a press briefing on April 17, 2023, Prof Mojisola Christianah Adeyeye, Director-General NAFDAC, said malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
According to the 2021 World Malaria Report, Nigeria had the highest number of global malaria cases (27%) and the highest number of related fatalities (32%) in 2020
This is the second authorization for R21/Matrix-M this month, following the Republic of Ghana.
GSK's Mosquirix™ RTS,S recombinant malaria vaccine was authorized in 2020.
Additional malaria vaccine and outbreak news are posted at Vax-Before-Travel.
INOVIO today announced that Dr. Angela Huttner, Infectious Disease Consultant, Geneva University Hospitals presented data from a Phase 1b trial evaluating INO-4201, an Ebola booster vaccine candidate for rVSV-ZEBOV (Ervebo®).
"Preliminary data showed that INO-4201 was well tolerated and produced a strong immune response," stated Dr. Huttner in a press release on April 17, 2023.
"This suggests that a booster dose of IN0-4201 has the potential to extend protection against Ebola and could be an important tool in future Ebola Virus Disease prevention."
In February 2023, INOVIO announced positive initial results from the Phase 1b trial that evaluated INO-4201 as a booster in healthy adult participants who previously received a single injection of Ervebo.
These initial results showed that INO-4201 was well-tolerated and boosted humoral responses in 100% (36 of 36) of treated participants. In addition, data presented today included the assessment of binding antibodies showing that all 36 vaccine recipients responded to the boost.
The unedited press release is posted at this link.
Note: Merck's Ervebo® Vaccine is a live, recombinant, replication-competent vaccine approved by the U.S. FDA and by the European Medicines Agency.
Since 2019, approximately 300,000 persons have been vaccinated with the Ervebo vaccine in Africa.
Other Ebolavirus (Zaire and Sudan) vaccine and outbreak(s) news is posted at Vax-Before-Travel.
Vaxcyte, Inc. today announced positive results from the VAX-24 Phase 2 study in adults aged 65 and older, as well as data from the full six-month safety assessment and prespecified pooled immunogenicity analyses from both the Phase 2 study in adults aged 65 and older and the prior Phase 1/2 study in adults aged 18-64.
VAX-24, the Company's lead, broad-spectrum 24-valent pneumococcal conjugate vaccine (PCV) candidate, is being studied to prevent invasive pneumococcal disease (IPD).
The Company says, 'The public health community continues to affirm the need for vaccines that offer broader protection to prevent IPD.'
"Based on the overall strength of our data and the well-established regulatory pathway, we look forward to meeting with regulators and advancing VAX-24 into a pivotal Phase 3 study for which we expect topline data in 2025," said Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte, in a press release on April 17, 2023.
"We developed VAX-24 to create a best-in-class PCV that provides broader coverage and better immune responses than standard-of-care vaccines."
"These data support that objective and demonstrate the potential of our PCV franchise, including VAX-31, our 31-valent PCV candidate."
In the Phase 2 study in adults aged 65 and older, VAX-24 demonstrated robust OPA immune responses for all 24 serotypes at all doses studied, confirming the prior adult study results.
The VAX-24 2.2mcg dose, which Vaxcyte plans to advance to Phase 3, showed an overall improvement in immune responses vs. PCV20 relative to the results from the prior Phase 2 study in adults aged 50-64.
And the six-month safety data from both studies showed safety and tolerability results for VAX-24 similar to PCV20 at all doses studied.
The unedited announcement is posted at this link.
Other pneumococcal (PCV13, PCV15, PCV20, PCV24) vaccine news is posted at Precision Vaccinations.