Search API
The journal Nature recently published a news article focused on an innovative tactic to reduce the number of dengue cases in Brazil.
This program aims to protect up to 70 million Brazilians from diseases such as dengue.
The non-profit World Mosquito Program announced it would release about five billion bacteria-infected mosquitoes annually in many of Brazil’s urban areas over the next ten years.
Researchers have tested the release of this type of mosquito, which carries a Wolbachia bacterium that stops mosquitoes from transmitting viruses. Wolbachia naturally infects about half of all insect species.
In Brazil, previous test results have been modest. For example, in Niterói, the intervention was associated with a 69% decrease in dengue cases, while in Rio de Janeiro, the reduction was 38%.
But this will be the first time that the technology is dispersed nationwide.
Nature posts the unedited article as of April 14, 2023.
In the U.S., the state of Florida launched a similar program in May 2021.
Florida continues to report the most travel-related and locally acquired dengue cases in the U.S. during 2023.
Globally, two dengue vaccines have been authorized.

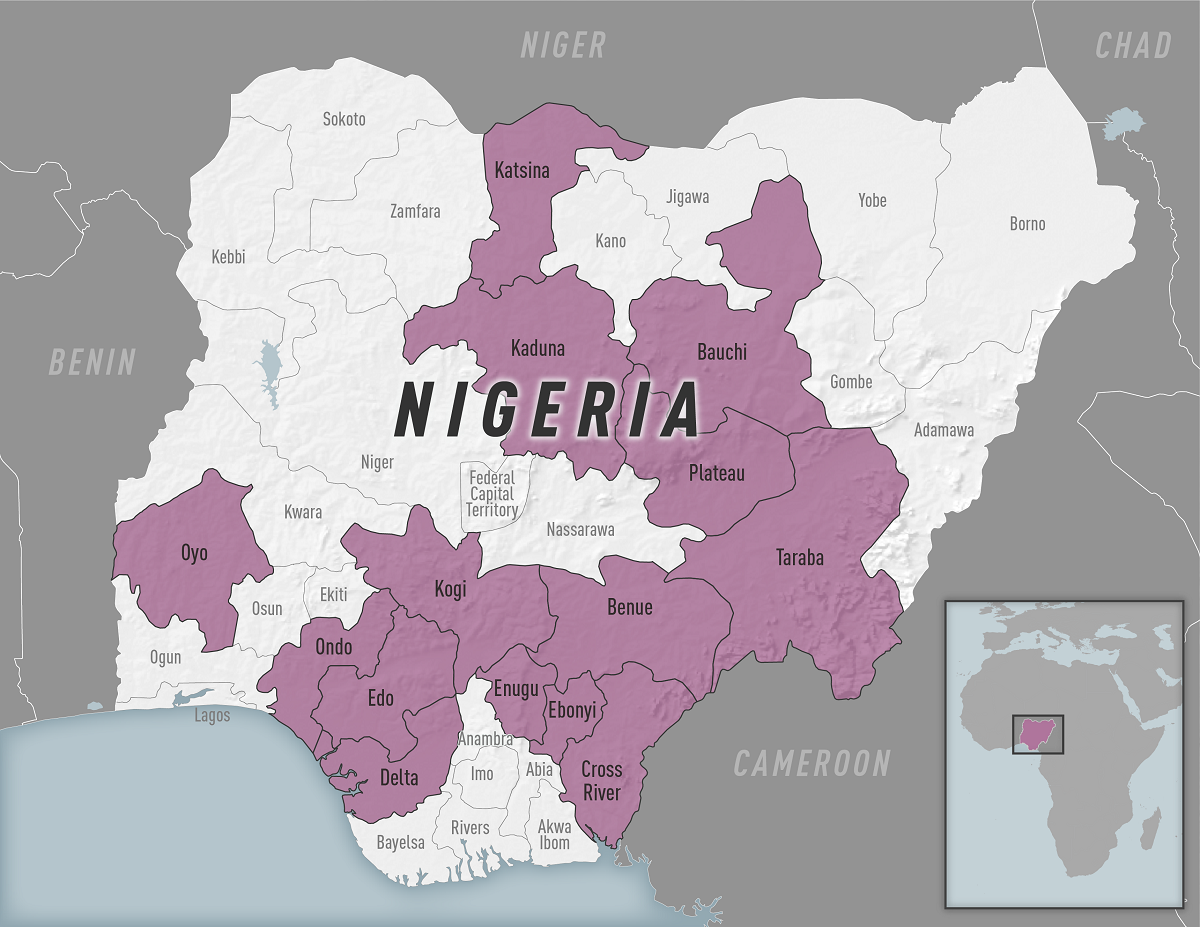
The Nigeria Centre for Disease Control and Prevention (NCDC) has reported an unprecedented negative trend in confirmed cases of Lassa Fever.
The NCDC recently confirmed a total of 869 confirmed Lassa Fever cases and 151 related fatalities from 26 states and 101 local government areas.
Cumulatively for 2023, the case fatality rate (CFR) of 17.4%.
As of April 9, 2023, for week #14 , the number of new confirmed cases is 23 from Bauchi, Ondo, Edo, Taraba, Lagos, and Enugu Niergian States.
Lassa fever is an acute viral hemorrhagic fever with a natural reservoir in the Mastomys natalensis rodent (African rat).
As of early March 2023, the U.S. CDC's Watch - Level 1, Practice Usual Precautions notice alerts visitors to Nirgeria of the health risk.
As of April 19, 2023, the U.S. FDA had not approved a Lassa fever vaccine.
AstraZeneca recently highlighted new data at the 33rd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), reinforcing its ambition to provide long-lasting immunity for millions globally.
For example, data featuring AZD3152, AstraZeneca’s investigational long-acting COVID-19 antibody, shows the investigational COVID-19 long-acting antibody neutralizes all known variants of concern identified to date.
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, stated in a press release on April 14, 2023, “Our first in vitro data from our next generation long-acting antibody, AZD3152, shows its potential to protect the immunocompromised from all known COVID-19 variants of concern to date.”
Last updated on April 3, 2023, the aim of the phase I/III clinical study is to evaluate the safety and neutralizing activity of AZD3152 compared with AZD7442 for pre-exposure prophylaxis of COVID-19 and separately evaluate the safety and PK of AZD5156, a combination of AZD3152 and AZD1061.
Other COVID-19 antibody news is posted by CoronavirusToday.com.

According to the U.S. government, pharmacies are the leading distribution outlet for COVID-19 vaccines. During the 2022-2023 season, available data show that more than two-thirds of adult COVID-19 vaccinations were administered at pharmacies.
Announced on April 18, 2023, the U.S. Department of Health & Human Services (HHS) announced the ‘HHS Bridge Access Program For COVID-19 Vaccines and Treatments Program (“Program”) to maintain broad access to COVID-19 vaccines for millions of uninsured Americans.
The program will create a unique $1.1 billion public-private partnership to help maintain uninsured individuals’ access to COVID-19 care at local pharmacies.
HHS wrote pharmacies had been a critical partner in the Administration’s response to COVID-19 and a vital access point for millions of Americans in receiving convenient and timely COVID-19 vaccines, treatments, and tests.
In the future, HHS aims to ensure that the pharmacy setting remains a place of access for the uninsured.
In building the Program, CDC will establish contracts with pharmacies to enable them to continue offering COVID-19 vaccines and designated treatments with no out-of-pocket costs to uninsured individuals, maintaining this critical access point for this population.
And pharmacies will also be expected to conduct outreach regarding the availability of the COVID-19 vaccine, including through community partnerships focusing on underserved populations.
Together, these efforts will create a unique public-private partnership that will help maintain uninsured individuals’ access to COVID-19 care at their local pharmacies, local health centers, and public health infrastructure.

The journal ScienceDirect recently published results from an early-stage, innovative Zika virus vaccine candidate, OraPro-Zika.
On April 6, 2023, this study addressed several challenging features attributed to vaccinology.
First, developing a thermostable vaccine negates the need for a "cold chain." And second, the potential to deliver the vaccine orally.
Having demonstrated that iosBio's OraPro-Zika vaccine provided a measurable efficacy in the murine models, they performed a challenge study in NHP to investigate the potential clinical utility.
In this setting, enteric-coated capsules were given orally, negating the need for stomach acid neutralization.
Upon vaccination, a significant rise in anti-Zika IgG was evident after the first dose concordant with our murine model.
Furthermore, the level of IgG was similar to that in convalescent serum from NHP previously infected with the Zika virus.
Most strikingly, in challenge studies, convalescent and OraPro-Zika vaccinated animals showed no evidence of replicative Zika virus. In contrast, the placebo-vaccinated cohort showed full-blown infection, which modeled the disease pathology as it was rectified by day 8 of the study.
While we hypothesize the immune protection in our model systems is potential via a mucosal root, our study is limited and does not define cellular mechanisms or sIgA.
Nonetheless, IgG and challenge studies remain the gold standard for vaccine efficacy.
In summary, these researchers showed that an orally administered Ad5- vaccine encoding genes for the Zika envelope (Env) and NS1 proteins induced a specific immune response that reduced Zika infection in both murine and NHP models and remained thermally stable for over 109 days at 25 °C.
This preliminary study with OraPro-Zika suggests oral administration of a non-replicating adenovirus vector protects against challenge and warrants further investigation to establish a mode of action.
These authors declared various industry relationships, and Innovate UK supported the study.
Updated May 10, 2023 - Reassigned domain.

The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Watch - Level 1, Practice Usual Precautions regarding dengue outbreaks in Asia and the Pacific Islands.
The CDC stated that on April 17, 2023, the listed countries reported higher-than-usual dengue cases, and travelers visiting these countries may be at increased risk.
Because Dengue is a severe disease spread by mosquito bites, all travelers to risk areas should prevent mosquito bites by using an EPA-registered insect repellent, wearing long-sleeved shirts and long pants outdoors, and sleeping in an air-conditioned room or room with window screens or under an insecticide-treated bed net.
The CDC says Dengue can take up to 2 weeks to develop, generally lasting less than a week.
Additionally, visitors to these countries should speak with a healthcare provider regarding dengue vaccines, such as Dengvaxia and Takeda's QDENGA®.
Vax-Before-Travel posts other dengue outbreak news.
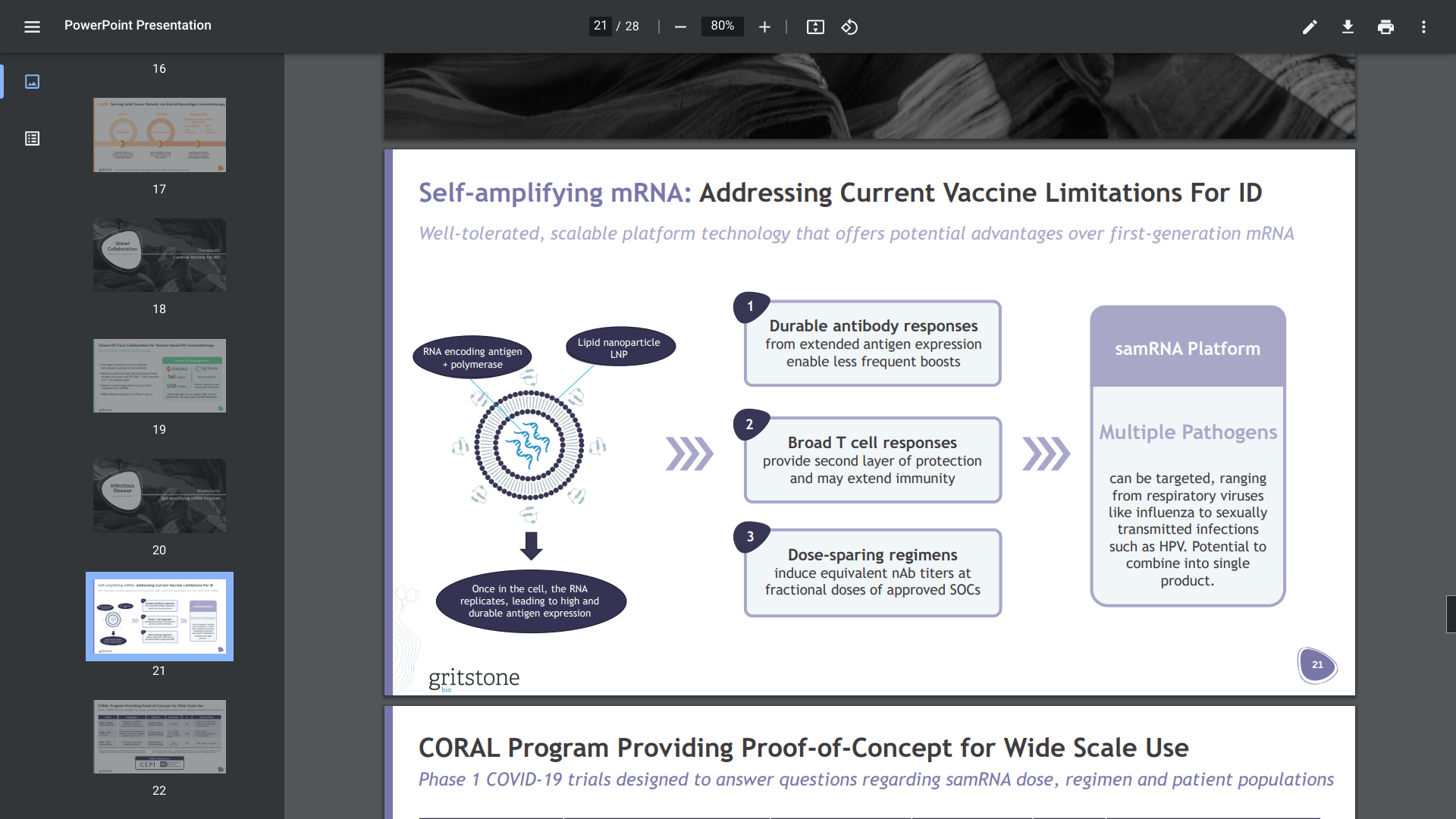
Gritstone bio, Inc. today presented 6-month follow-up data from its ongoing Phase 1 CORAL-CEPI and CORAL-BOOST studies, which are evaluating the company's self-amplifying mRNA (samRNA) vaccine candidates against SARS-CoV-2, at the 33rd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
"The results shared today demonstrate that regardless of whether and/or how the immune system was previously exposed to SARS-CoV-2, our samRNA vaccine candidates are driving robust antibody titers that are sustained for at least six months in healthy adults," said Karin Jooss, EVP and Head of R&D at Gritstone bio, in a press release on April 17, 2023.
"Current vaccines against COVID-19 have demonstrated susceptibility to loss of immunity over time, posing a greater burden on individuals and our health systems."
"Developing a next-generation vaccine that drives more durable and broad neutralizing antibodies against variants of concern not included in the vaccine – both of which are demonstrated in these results - could serve as a key factor in delivering long-term, variant-proof immune protection."
"The results shared today further support our hypothesis that samRNA, unique from mRNA due to several distinct characteristics, could serve as a widely applicable next-generation vaccine platform technology against SARS-CoV-2 and beyond."
Gritstone's CORAL program applies an infectious disease approach, which aims to drive B cell and T cell immunity using samRNA against SARS-CoV-2.
The program serves as proof of concept for applying Gritstone's platform against coronaviruses and other infectious diseases. The Bill & Melinda Gates Foundation, the U.S. NIAID, and the Coalition for Epidemic Preparedness Innovations support it.
SAB Biotherapeutics announced today that the U.S. Food and Drug Administration (FDA) had granted Breakthrough Therapy Designation to SAB-176, an investigational therapeutic, for post-exposure prophylaxis for Type A and Type B influenza illness in high-risk patients, including those who have antiviral resistant strains.
SAB-176 is being developed for several influenza indications.
The FDA's Breakthrough Therapy designation confirms that the multi-epitope targeting modality of SAB-176 has a clear differentiation vs. monoclonal antibodies (mAbs) that bind to a single epitope.
And SAB's treatment can sustain its efficacy over viral mutations and prevent or reduce the risk of emerging treatment-resistant influenza strains.
Virus evolution driven by vaccines or treatments is a serious challenge, and the use of therapeutics can create "escape mutants" or versions of a virus that have changed to escape pressure on virus survival driven by antiviral treatment, whether it is a small molecule or mAbs modality, wrote the company.
SAB recently announced that the FDA had granted Fast Track designation to SAB-176.
The company had also received FDA guidance and regulatory alignment on advancing SAB-176 into the next development phase by initiating a Phase 2b clinical trial.
"Influenza continues to pose considerable health concerns in the U.S. and globally. This Breakthrough Therapy designation signifies an important step forward in our fight against this disease," said Eddie Sullivan, Ph.D., co-founder, President & CEO of SAB Biotherapeutics, in a press release on April 18, 2023.
"We are proud that based on generated preclinical and clinical evidence, SAB-176 has received both Breakthrough and Fast Track designations, a combination rarely seen."
The FDA's Breakthrough Therapy designation process is designed to expedite the development and reviewing a medicine intended to treat a serious or life-threatening condition. Preliminary clinical evidence indicates that the drug may substantially improve over current therapies on a clinically significant endpoint(s).
Products that qualify for Breakthrough Therapy designation receive more benefits than Fast Track products.
Precision Vaccinations post influenza vaccines news for April 2023.