Search API
Novavax Inc. today announced the full results from the pediatric expansion of the Phase 3 PREVENT-19 clinical trial were published in the Journal of the American Medical Association Network Open on April 26, 2027.
The study's expansion evaluated the safety and effectiveness of Novavax's COVID-19 prototype vaccine (NVX-CoV2373) in adolescents aged 12 through 17 across the U.S.
Novavax's U.S. Food and Drug Administration-approved vaccine achieved its primary effectiveness and efficacy endpoint in the trial when the Delta variant was the predominant circulating SARS-CoV-2 coronavirus strain.
There was no increase in reactogenicity in younger (12 to <15 years old) adolescents compared to older (15 to <18 years old) adolescents.
Non-inferior neutralizing antibody responses compared to young adults in the main adult study were demonstrated, which was the critical regulatory endpoint for authorization.
And safety data showed the vaccine to be generally well-tolerated in the placebo-controlled portion of the study.
As of April 27, 2023, Novavax protein-based COVID-19 vaccines have been authorized in more than 30 markets worldwide, known as Nuvaxovid™, CovoVax™, and NVX-CoV2373.

The World Health Organization (WHO) today published Edition #140 of its Weekly epidemiological update on the COVID-19 pandemic.
As of April 27, 2023, the WHO confirmed nearly 2.8 million new COVID-19 cases, and over 16,000 related fatalities were reported in the last 28 days.
This data indicates a decrease of 23% and 36%, respectively, compared to the previous period.
Contrary to the overall trend, increases in reported cases and deaths continued to be registered in the South-East Asia and Eastern Mediterranean regions and several individual countries elsewhere.
Merck today announced financial results for the first quarter of 2023. In addition, Merck stated it realized lower sales of the oral COVID-19 antiviral Lagevrio™ (Molnupiravir), which decreased 88% to $392 million.
This decrease was primarily attributable to sales in the U.S. and U.K. markets in the first quarter of 2022 that did not recur in the first quarter of 2023.
LAGEVRIO's decline was also attributable to lower sales in Japan and Australia.
LAGEVRIO is approved or authorized for use in more than 25 countries. It helps reduce how sick people become when diagnosed with COVID‑19.
Additional COVID-19 antiviral news is posted at Coronavirus Today.
GSK plc today announced that the European Medicines Agency's Committee for Medicinal Products for Human Use had adopted a positive opinion by consensus recommending approval of GSK's respiratory syncytial virus (RSV) vaccine candidate for the prevention of lower respiratory tract disease caused by RSV in adults aged 60 years and older.
If approved, AREXVY™ RSV OA candidate has the potential to be the first RSV vaccine available to help protect older adults.
The European Commission's final decision is expected by July 2023.
This is the first time an RSV vaccine candidate for adults has gained a positive opinion, one of the final steps in the marketing authorization procedure before approval by the European Commission.
As of April 27, 2023, no RSV vaccines or specific treatments are currently available for older adults in Europe or the U.S.
RSV is a common contagious virus affecting the lungs and breathing passages. RSV causes over 270,000 hospitalizations and approximately 20,000 in-hospital deaths in adults aged 60 years and older each year in Europe.
However, according to recent information, RSV's intensity may have returned to normal in the U.S.
The U.S. CDC's Morbidity and Mortality Weekly Report, published on April 7, 2023, presented the seasonality of RSV in the U.S. from 2017–2023.
The CDC reported the 2022–23 RSV season started later than the 2021–22 season but earlier than the prepandemic seasons, suggesting a return toward prepandemic seasonality.
For updated information, the CDC's RSV-NET interactive dashboard displays trends and comparisons of RSV-associated hospitalizations in various demographic groups and seasons.

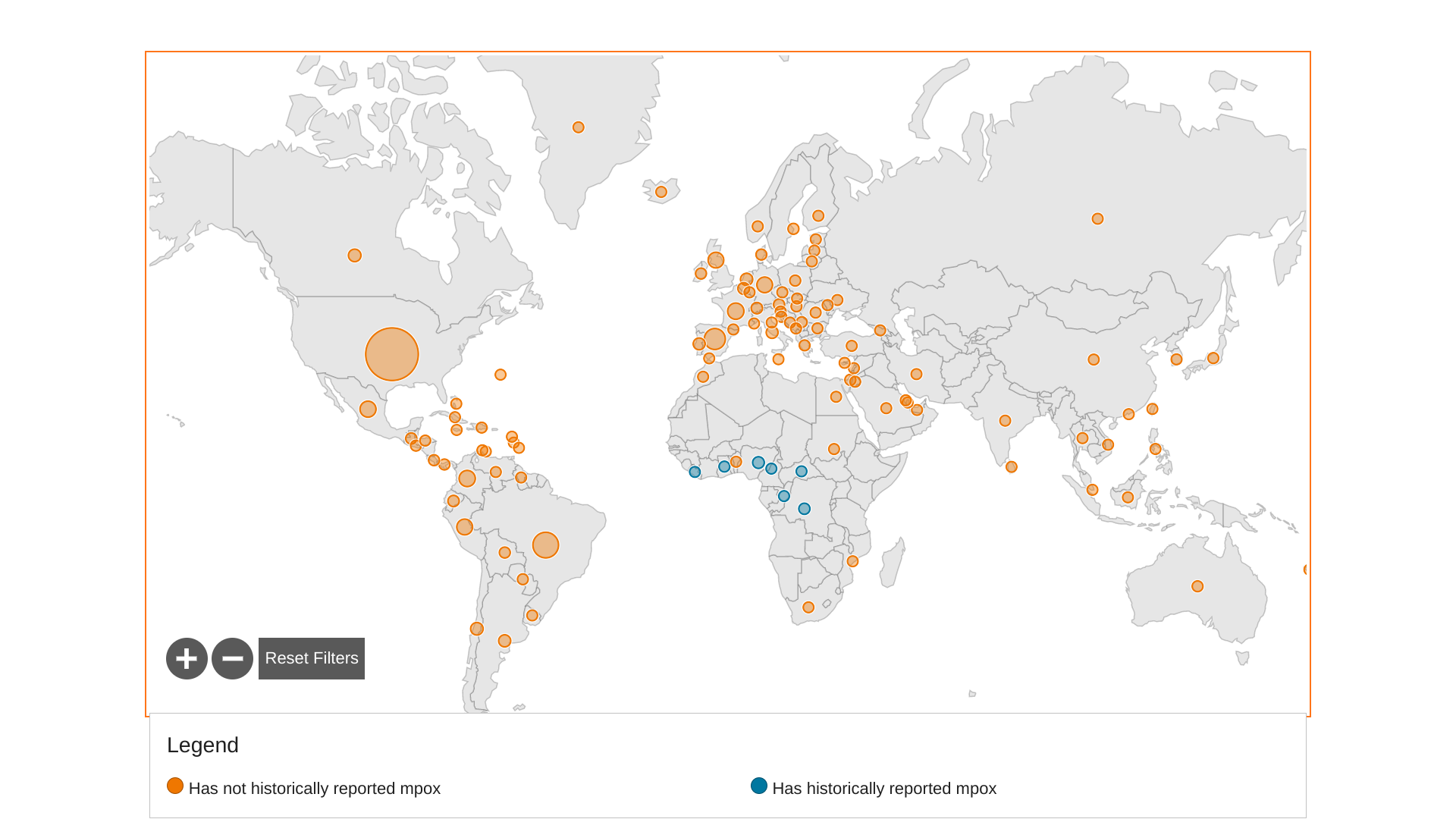
The World Health Organization Africa Region recently reported Mpox cases in the Central Africa Republic, the DRC, Ghana, Liberia, and Nigeria.
As of April 16, 2023, fourteen newly confirmed Mpox cases were reported, resulting in a 5.6% increase.
And three new Mpox-related fatalities were reported.
The top three African countries with the most confirmed Mpox cases include Nigeria, 833, the DRC 450, and Ghana, 124. Together, the three countries have reported 93.7% of all confirmed Mpox cases in Africa.
To alert international travelers of potential health risks when visiting Africa, the U.S. CDC has published Watch - Level 1, Practice Usual Precautions notices for the DRC and Nigeria.
Globally, over 86,000 Mpox cases have been reported by 110 countries since the outbreak began in May 2022.
While the Mpox outbreak has receded in the U.S., the CDC recommends Bavarian Nordic's JYNNEOS® (MVA-BN) vaccination when visiting at-risk countries in 2023.
Additional Mpox outbreak research is posted at Precision Vaccinations.
Based on a measles outbreak declaration by American Samoa Government, travelers from the Territory (including infants aged six months) must be vaccinated with a measles-containing vaccine before entering Samoa.
Being fully vaccinated indicates completing the required doses, which should have been completed 14 days before traveling.
These islands are located in the South Pacific Ocean, northeast of Fiji.
And Aiono Prof Alec Ekeroma, Samoa's Director General of Health, stated in a Notice that effective May 1, 2023, a legitimate vaccine certificate/note is required before boarding. In addition, a hard copy must be presented at check-in and upon arrival into Samoa.
Vaccine certificates in electronic form stored on phones or other electronic devices must have a QR code. In addition, they must be a Health Authority Approved Certificate/Card of the country where the vaccination occurred.
Furthermore, upon arrival in Samoa, all passengers must wear face masks at all times.
And visitors must submit to a nasal pharyngeal swab for PCR testing upon request by Health Officials at Samoa's airport.
As of April 26, 2023, the U.S. CDC recommends various routine and travel vaccinations before visiting America Samoa, an unincorporated territory of the U.S.
Prior to the recent pandemic, about 20,000 people visited America Samoa annually.
Bloomberg Law News recently reported that Chinese foreign ministry spokeswoman Mao Ning confirmed beginning April 29, 2023, China will no longer require visitors to provide a negative PCR test result.
Instead, visitors can show negative rapid antigen test results, and airlines will not be required to check for proof.
China previously eliminated testing requirements for countries such as New Zealand and Malaysia.
In 2023, the U.S. Centers for Disease Control and Prevention (CDC) began requiring a negative COVID-19 test result or proof of recovery from the virus for all travelers aged two years and older to the U.S. on flights originating from the People's Republic of China (PRC),
As of April 26, 2023, the U.S. CDC recommends various routine and travel vaccinations before visiting the PRC.
Previously, the U.S. Department of State issued a notice stating 'reconsider travel' to the PRC, including the Special Administrative Regions of Hong Kong and Macau, due to arbitrary enforcement of local laws.