Search API
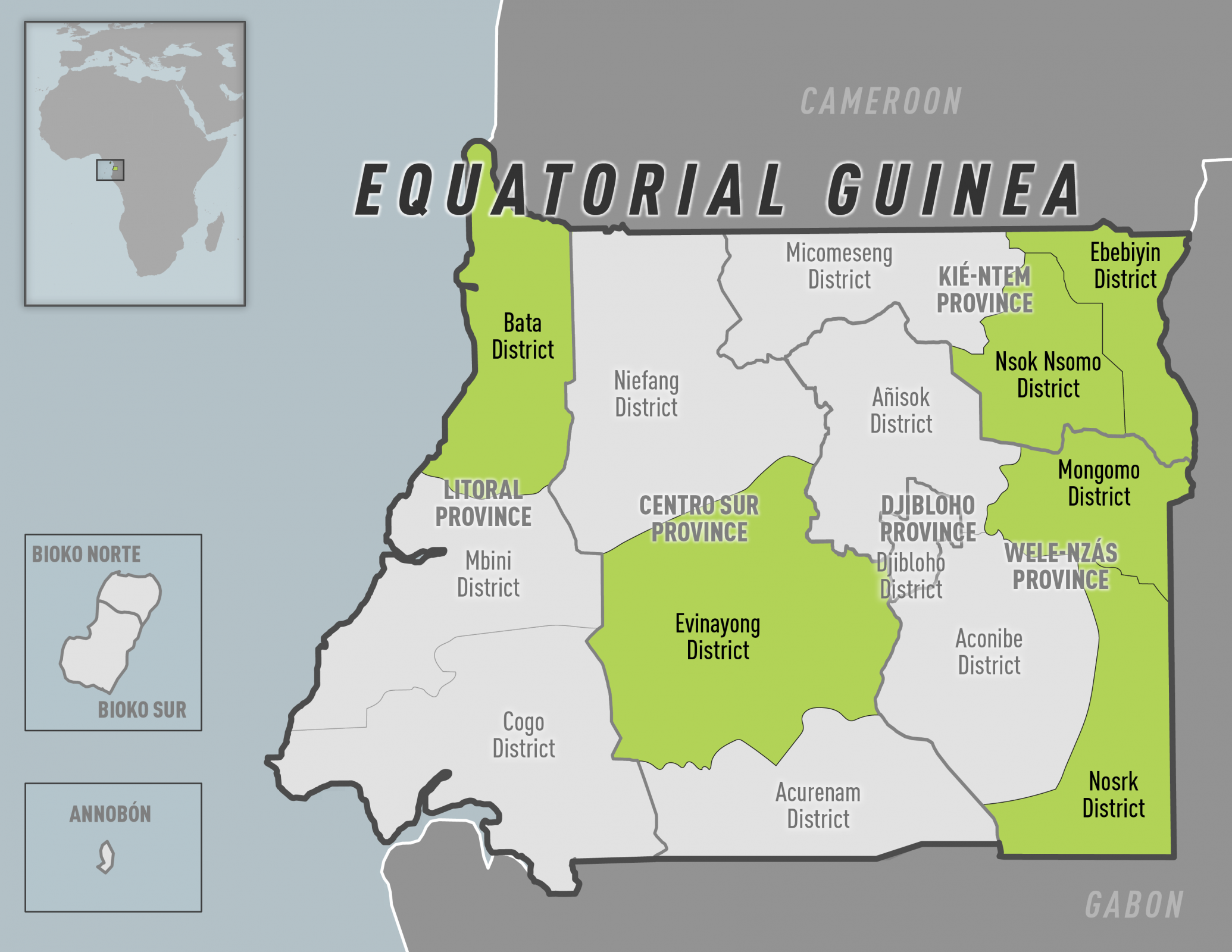
The U.S. Centers for Disease Control and Prevention (CDC) reissued its Alert - Level 2, Practice Enhanced Precautions notice regarding the ongoing Marburg virus disease (MVD) outbreak in the Republic of Equatorial Guinea.
As of May 12, 2023, the CDC says, 'Reconsider non-essential travel to mainland Equatorial Guinea.'
If you travel to Equatorial Guinea, you should:
- Avoid contact with sick people with fever, muscle pain, and rash symptoms.
- Avoid contact with blood and other body fluids.
- Avoid contact with dead bodies or items that have been in contact with dead bodies, participate in funeral or burial rituals, or attend a funeral or burial.
- Avoid visiting healthcare facilities in the outbreak area for nonurgent medical care or non-medical reasons.
- Avoid visiting traditional healers.
- Avoid contact with fruit bats and the caves and mines where they live.
- Avoid nonhuman primates (e.g., chimpanzees, gorillas).
Furthermore, visitors should watch their health for symptoms of Marburg, a viral hemorrhagic fever, while in the outbreak area and 21 days after leaving the outbreak area.
As of May 14, 2023, other Marburg outbreak alerts have been issued this year for Africa.
Unfortunately, there are no Marburg vaccines available.
The U.S. Centers for Disease Control and Prevention (CDC) today announced endemic transmission of wild poliovirus type 1 (WPV1) continues only in Afghanistan and Pakistan.
As of May 12, 2023, the CDC reported Malawi and Mozambique confirmed WPV1 cases linked to a Pakistan strain in 2022.
These cases are the first WPV1 cases in the African region since 2016.
During the last week, these countries reported polio findings:
- Pakistan: two WPV1-positive environmental samples
- Chad: one cVDPV2 case
- DR Congo: six cVDPV2 cases
- Madagascar: 17 cVDPV1 positive environmental samples
- Nigeria: three cVDPV2 cases and two cVDPV2 positive environmental samples
- Somalia: one cVDPV2 case
Furthermore, the total number of samples collected in countries with poliovirus transmission increased from 8,945 in 36 countries in 2021 to 12,259 samples from 40 countries in 2022.
The detections of poliovirus in areas where it had been previously eliminated, such as New York, underscore the threat of continued poliovirus spreading to any place where the population is insufficiently vaccinated against it, wrote the CDC.
The CDC reissued an Alert - Level 2, Practice Enhanced Precautions regarding polio outbreaks to notify international travelers of this health risk.
Various polio vaccines are available globally and in the U.S. at clinics and community pharmacies.

Local media recently reported a case of Ebola virus disease in The Democratic Republic of Congo (DRC) on May 8, 2023. The last Ebola outbreak in the DRC occurred in 2022.
“Regarding Ebola virus disease surveillance, we received a sample that turned out to be positive from the Kyondo health zone and in the Butembo site. We have received four samples, and among the four, one is positive,” commented Damulo Luhavo, the communicator of the provincial health division of Butembo.
Luhavo called on the local population to continue to be vaccinated.
As of May 13, 2023, various Ebola vaccines and Monoclonal Antibody therapies are available.
This news article did not disclose which type of Ebola, Sudan or Zaire, was detected.
The U.S. Centers for Disease Control and Prevention (CDC) confirmed today, May 12, 2023, that noncitizen nonimmigrant air passengers no longer need proof of being fully vaccinated with an accepted COVID-19 vaccine to board a flight to the United States.
An announcement of the termination of this U.S. government's Amended Order was published in the Federal Register.
Additionally, the CDC today published revised face mask guidance for air travelers.
The CDC says when people wear high-quality face masks, they protect themselves and those around them and help keep travel safer.
These CDC announcements should increase airport passenger activity, which is running about 95% of what the U.S. TSA screened in 2019.

CNA reported yesterday another young woman living in Taiwan was diagnosed with the A H1N2 variant (H1N2v) of the novel swine influenza virus.
As of May 11, 2023, this is only the third swine flu case ever seen in Taiwan, according to the Centers for Disease Control.
This zoonotic influenza case came into contact with swine from working at a pig farm. But the virus was not detected in any of the pigs on the farm where the girl worked.
Close contacts were identified, but none tested positive for the H1N2v virus.
Taiwan's first human H1N2v infection was reported in April 2021.
Unlike swine flu, avian influenza Highly Pathogenic Avian Influenza (HAPI) viruses have been detected in birds, mammals (cats, bears, dogs), and humans during 2022-2023.
The U.S. Centers for Disease Control and Prevention (CDC) Technical Report issued on March 17, 2023, confirmed the current risk to the public from HPAI A(H5N1) viruses remains low.
However, continued sporadic human infections are anticipated because of the potential for influenza viruses to evolve.
As of April 6, 2023, about 240 cases of human infection with avian influenza A(H5N1) virus have been reported from four countries within the Western Pacific Region since January 2003.
Of these bird flu cases, 135 were fatal, resulting in a case fatality rate of 56%.
Should a human pandemic occur in 2023, the U.S. government has already stockpiled U.S. FDA-approved bird flu vaccines, and other development efforts have been funded as of May 12, 2023.
Furthermore, the CDC says annual flu shots are not designed to protect people against either bird or swine flu infections.

Data recently published by the U.K. Health Security Agency (UKHSA) shows a rise in measles cases related to under-vaccinations in England.
Between January and April 2023, there have been 49 measles cases confirmed in the U.K. compared to 54 for all of 2022.
Most measles cases have been in London; some are linked to international travel.
The measles virus is easy to catch in closed environments such as airplanes.
In recent years, the number of children vaccinated against measles has fallen in England, says the UKHSA on May 4, 2023.
The uptake for the first dose of the MMR vaccine in children is about 89%.
Measles infections can lead to severe problems such as pneumonia. Symptoms include a high fever, sore red, watery eyes, and a blotchy red-brown rash.
Measles cases worldwide increased by about 80% during 2022 compared with 2021.
To alert international travelers, the U.S. CDC published a global Watch-Level 1, Practice Usual Precautions notice on April 6, 2023, regarding measles outbreaks in various countries.
The CDC's top ten global measles outbreaks, as of May 2023, were led by India, with about 68,000 cases.
In the U.S., measles vaccines are generally available at clinics and community pharmacies.
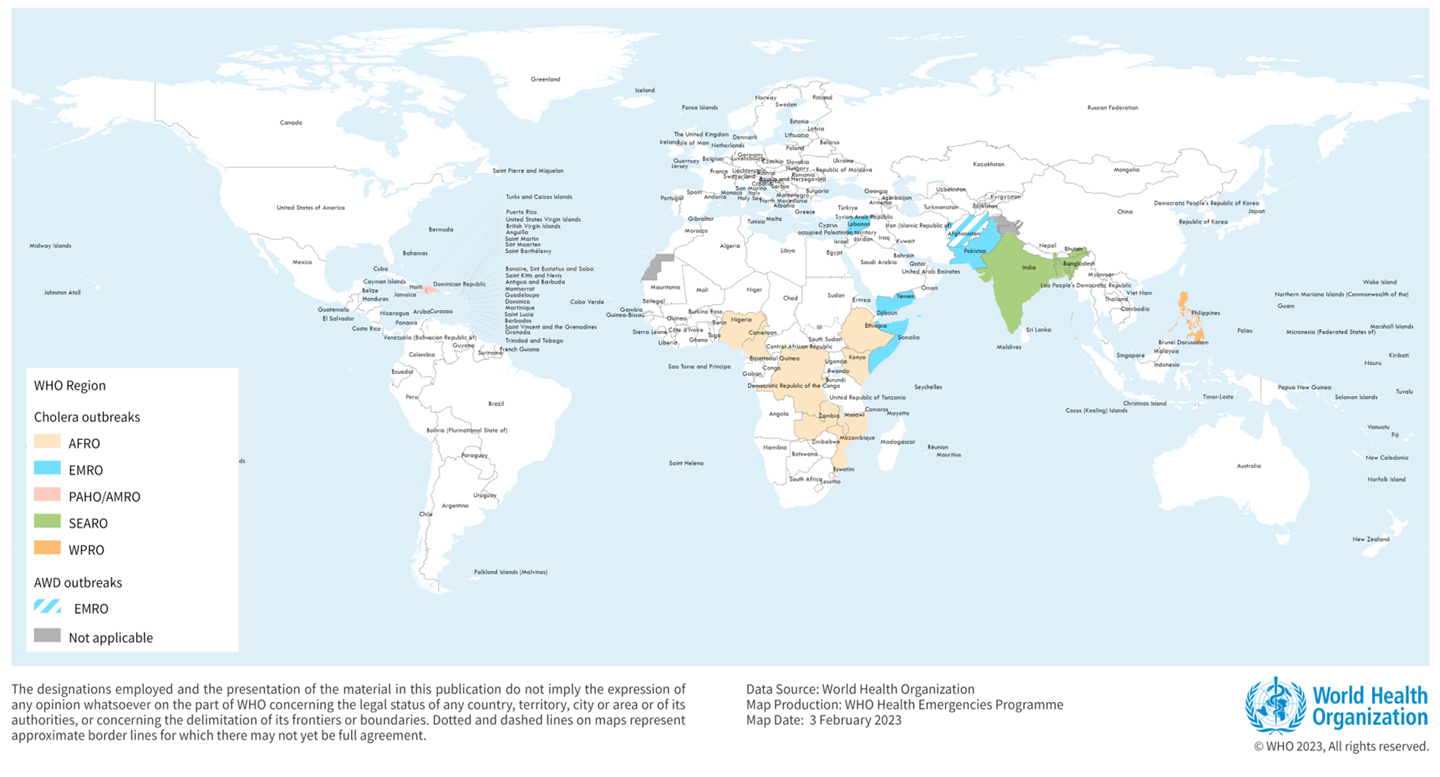
The World Health Organization (WHO) External Situation Report #2 confirmed yesterday that the Kingdom of Eswatini reported its first cholera case in April 2023, related to cross-border transmission from Mozambique and a second case without travel history.
In total, 24 countries have reported cholera cases since the beginning of 2023.
The overall capacity to respond to multiple and simultaneous outbreaks continues to be strained due to the global lack of resources, including shortages of the oral cholera vaccine.
Based on the current situation, the WHO assessed on May 11, 2023, the risk at the global level is very high.
Cholera outbreak news is posted by Vax-Before-Travel.
Before visiting Eswatini in southeast Africa, the U.S. CDC suggests various travel vaccinations, including yellow fever.
GSK plc today presented preliminary positive results from the phase III trial evaluating the immunological vaccine effectiveness and safety of its MenABCWY combination vaccine candidate.
On Mau 12, 2023, the vaccine candidate demonstrated non-inferiority in primary endpoints for five Neisseria meningitidis serogroups (A, B, C, W, and Y) compared to two doses of Bexsero® (meningococcal group B vaccine) and one dose of Menveo (meningococcal group A, C, W-135, and Y conjugate vaccine) in 10–25-year-olds.
In addition, the vaccine candidate was generally well tolerated, with a safety profile consistent with Bexsero and Menveo.
In a separate confirmatory arm of this phase III trial, the MenABCWY vaccine candidate showed immunological effectiveness against a panel of 110 diverse meningococcal serogroup B (MenB) invasive strains, which account for 95% of strains circulating in the US.
Professor Terry Nolan, principal investigator for the phase III trial and Head of the Vaccine and Immunisation Research Group at the Peter Doherty Institute for Infection and Immunity at the University of Melbourne and Murdoch Children's Research Institute, said in a press release, "Meningococcal vaccination can help save lives, and these results are significant in moving one step closer to protection against five meningococcal serogroups with a single vaccine."
"The potential for a simplified immunization schedule could improve accessibility for the target population susceptible to meningococcal disease."
Five Neisseria meningitidis serogroups (A, B, C, W, and Y) account for nearly all invasive meningococcal disease (IMD) cases worldwide.
Meningitis B is the most common serogroup in the US, accounting for more than half of meningococcal disease cases among 16–20-year-olds.
Currently, immunization coverage rates for Men B are estimated at approximately 31% of adolescents in the US.
GSK stated it is working closely with regulatory agencies to review the complete phase III data set, including the supplemental Biologics License Application for Bexsero, to confirm full licensure under the Accelerated Approval pathway. Detailed results will be submitted for publication in a peer-reviewed scientific journal later in 2023.
Precision Vaccinations publishes meningococcal vaccine news.

Data published by Sanofi today from the HARMONIE Phase 3b clinical trial show an 83.21% (95% CI 67.77 to 92.04; P<0.001) reduction in hospitalizations due to RSV-related lower respiratory tract disease (LRTD) in younger infants who received a single dose of Beyfortus® (Nirsevimab).
While the U.S. Food and Drug Administration approved a multi-dose, injectable monoclonal antibody (mAbs) therapy in 1998, this new therapy has been authorized in Canada, Europe, and the U.K. as of May 12, 2023.
The Hospitalized RSV Monoclonal Antibody Prevention (HARMONIE) study is a large (8,000), multi-country interventional clinical trial aiming to determine the efficacy and safety of a single intramuscular dose of nirsevimab, with data collected in a real-world setting during the 2022-2023 RSV season.
The data from HARMONIE also show that nirsevimab reduced the incidence of hospitalizations due to severe RSV-related LRTD (patients whose oxygen level is under 90% and require oxygen supplementation) by 75.71% (95% CI 32.75 to 92.91; P<0.001).
Additionally, nirsevimab demonstrated a reduction of 58.04% (95% CI 39.69 to 71.19; P<0.001) in the incidence of all-cause LRTD hospitalization compared to infants who received no RSV intervention.
Throughout HARMONIE, nirsevimab maintained a favorable safety profile, consistent with the pivotal trial results.
In a press release, Dr. Simon Drysdale, Consultant Pediatrician in Infectious Diseases at St. George’s University Hospital NHS Foundation Trust and Co-Chief Investigator of HARMONIE, stated, “RSV-related chest infections lead to high numbers of infants under 12 months old being hospitalized every year."
"These data reinforce the potential public health benefit of nirsevimab in helping reduce the strain on hospitals caused each year by RSV.”
Additional RSV vaccine and monoclonal antibody news are posted by Precision Vaccinations.

