Search API
Pfizer Inc. today announced that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted that the available data support the efficacy and safety of its unadjuvanted bivalent respiratory syncytial virus (RSV) prefusion F vaccine candidate RSVpreF or PF-06928316 (ABRYSVO™).
The vaccine candidate is under FDA review for preventing medically attended lower respiratory tract disease (MA-LRTD) and severe MA-LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant women.
The VRBPAC voted 14 to 0 on effectiveness and 10 to 4 on safety.
“We are encouraged by the outcome of today’s VRBPAC meeting as it is a critical step forward in the scientific community’s long-sought-after goal to help prevent RSV disease in infants during their most vulnerable first six months of life,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a related press release.
Additional RSV vaccine and monoclonal antibody news are posted by Precision Vaccinations.

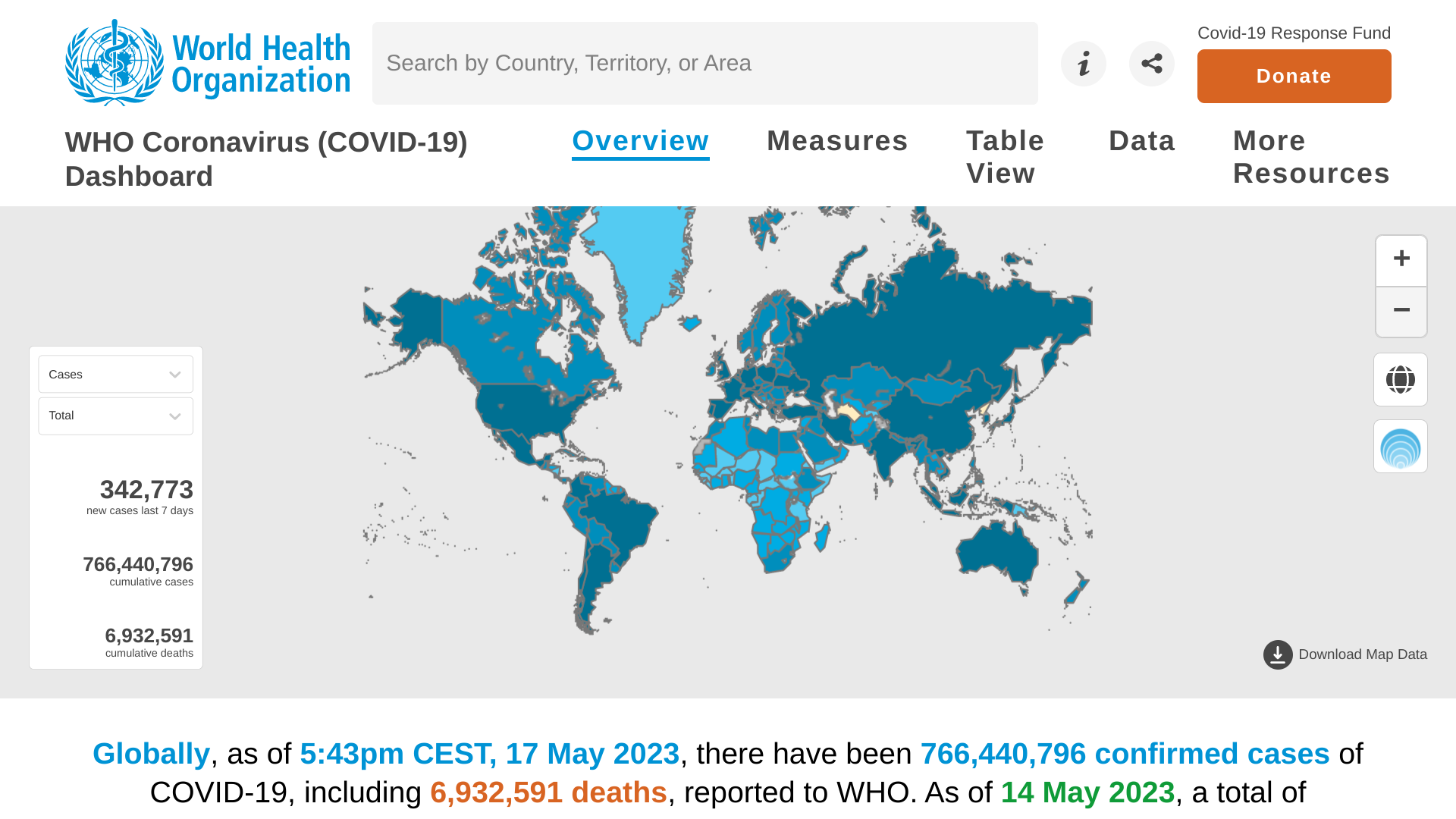
The World Health Organization (WHO) today announced nearly 2.6 million new COVID-19 cases, and over 17,000 deaths were reported in the last 28 days, a decrease of 14% and 26%, respectively, compared to the previous 28 days.
Weekly epidemiological update #143 was published on May 18, 2023, and stated the COVID-19 situation is mixed at regional levels, with increases in reported cases in South-East Asia and Western Pacific regions and increases in deaths in South-East Asia.
At the country level, the highest numbers of new 28-day cases were reported from the Republic of Korea (418, 960 new cases; +46%), the United States of America (-34%), Japan (+15%), India (+32%), and Brazil (-28%).
The U.K. Health Security Agency (UKHSA) recently detected influenza A (H5) virus in two poultry workers who have recently worked on an infected poultry farm in England.
Neither person has experienced any avian influenza (bird flu) symptoms, and both have since tested negative.
Professor Susan Hopkins, Chief Medical Advisor at UKHSA, stated in a press release on May 16, 2023, "Current evidence suggests that the avian influenza viruses we're seeing circulating in birds around the world do not spread easily to people."
"However, we know already that the virus can spread to people following close contact with infected birds, and this is why, through screening programs like this one, we are monitoring people who have been exposed to learn more about this risk."
"Globally, there is no evidence of the spread of this strain from person to person, but we know that viruses evolve all the time, and we remain vigilant for any evidence of changing risk to the population."
"It remains critical that people avoid touching sick or dead birds."
In the U.S., one bird flu vaccine is approved by the U.S. FDA.
Precision Vaccinations posts updated news on the global avian influenza outbreak in birds, mammals, and humans.

The World Health Organization (CDC) Technical Advisory Group for COVID-19 Vaccine Composition today announced its advice on the composition of future formulations of COVID-19 vaccines.
The objective of an update to COVID-19 vaccine antigen composition is to enhance vaccine-induced immune responses.
Updating the vaccine composition considers the evolution of the SARS-CoV-2 beta coronavirus variants and aims to improve protection against symptomatic disease.
The Group suggests that future formulations of COVID-19 vaccines use newer variants in their composition, i.e., XBB.1 descendant lineages.
As of May 2023, the XBB.1 descendent lineages currently predominate globally (i.e., XBB.1.5, XBB.1.16, XBB.1.9).
Furthermore, estimates of vaccine efficacy (VE) against currently circulating SARS-CoV-2 variants, including XBB.1 descendent lineages, are very limited in terms of the number of studies, vaccine products evaluated, and populations assessed; some studies show similar VE against BA.5 descendent and XBB.1 descendent lineages, while others suggest reduced VE during periods of the predominance of XBB.1 descendent lineages.
Additionally, the TAG-CO-VAC continues to encourage the further development of vaccines that enhance mucosal immunity because they may improve protection against infection and reduce transmission of SARS-CoV-2, in alignment with the WHO Global COVID-19 Vaccination Strategy, published in July 2022.
On May 18, 2023, the WHO stated the current COVID-19 vaccines continue to be highly protective against severe disease and death.
And the WHO strongly encourages the use of available authorized COVID-19 vaccines, which include the index SARS-CoV-2 virus, according to recommendations from the Strategic Advisory Group of Experts on Immunization, updated in March 2023.

During the U.S. Centers for Disease Control and Prevention COCA Call today, experts confirmed new mpox cases have occurred in some vaccinated men in Chicago, Illinois.
These mpox patients were vaccinated less than one year ago.
On side #46, the COCA call presented from March 18 through May 15, 2023:
- 21 men reported mpox infections to the Chicago Dept. of Public Health,
- 17 cases (of 21 with information) were vaccinated,
- 11 with two doses of the JYNNEOS® vaccine, 5 with one dose, and 1 with the smallpox vaccine ACAM2000,
- 5 had well-controlled HIV,
- And none were hospitalized.
'It is important that clinicians quickly identify cases to limit a possible mpox resurgence this summer in the United States,' wrote these CDC experts.
A replay of the COCA Call on May 18, 2023, will be accessible on this CDC webpage https://emergency.cdc.gov/coca/calls/2023/callinfo_051823.asp
The U.S. Food and Drug Administration (FDA) today published the Briefing Document for the Vaccines and Related Biological Products Advisory Committee (VRBPAC) review of ABRYSVO™, a respiratory syncytial virus (RSV) vaccine.
Pfizer Inc.'s ABRYSVO is a bivalent vaccine candidate comprised of two preF proteins selected to optimize protection against RSV A and B.
This digital meeting is scheduled for May 18, 2023, and starts at 8:30 AM ET and is open to the public.
The VRBPAC provides independent expert advice to the FDA on broad scientific topics or certain products to help the agency make sound decisions based on the available science.
GSK's AREXVY™ RSV OA single-dose RSV vaccine was previously approved by the FDA for seniors.
Furthermore, there are several other RSV vaccine candidates conducting late-stage studies.
Update May 18, 2023 - EXECUTIVE SUMMARY - This document summarizes the favorable benefit-risk profile for Pfizer’s RSVpreF (Abrysvo), a bivalent respiratory syncytial virus (RSV) stabilized prefusion F subunit vaccine (RSVpreF) for the proposed indication for prevention of lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants from birth through 6 months of age, by active immunization of pregnant individuals.

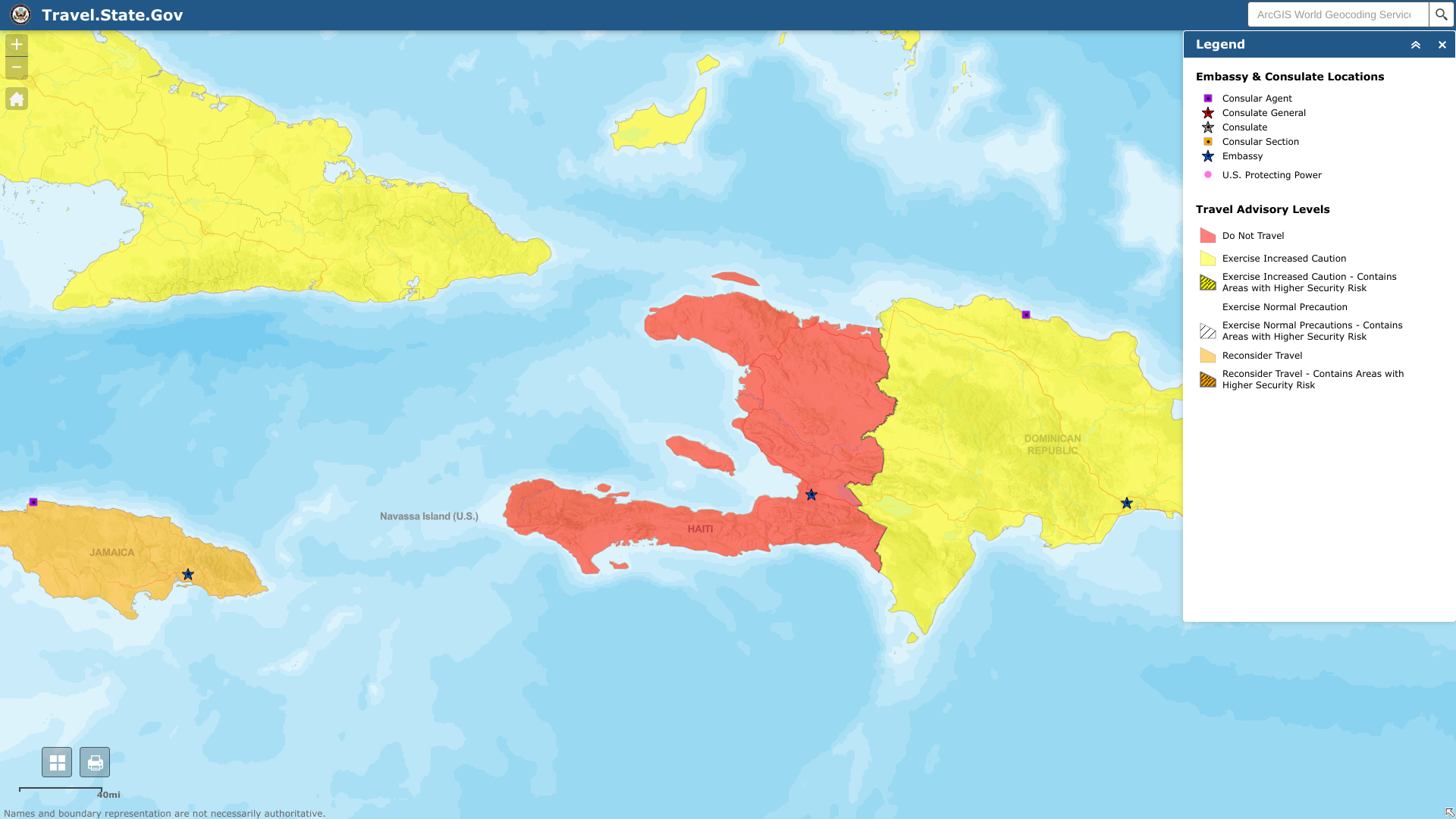
The U.S. Department of Stated today reissued its Level 4: Do Not Travel for the Republic of Haiti due to civil unrest.
Announced on May 17, 2023, the Department of States says U.S. citizens in Haiti should consider departing Haiti by commercial or other privately available transportation options in light of the current security situation and infrastructure challenges.
The U.S. Embassy in Port-au-Prince suspended employee travel to Cap Haitien from May 17-21, 2023.
And U.S. citizens wishing to depart Port-au-Prince should monitor local news and only do so when considered safe.
Furthermore, U.S. government personnel are discouraged from walking in Port-au-Prince.
And only family members over the age of 18 are permitted to accompany U.S. government employees assigned to the U.S. Embassy in Port-au-Prince.
From a health perspective, the Haitian Ministry of Health and Population recently confirmed an ongoing cholera outbreak.
Additionally, the U.S. CDC recommends various travel vaccinations, such as typhoid and yellow fever.
Other Disease Hot Spots are posted by Vax-Before-Travel.
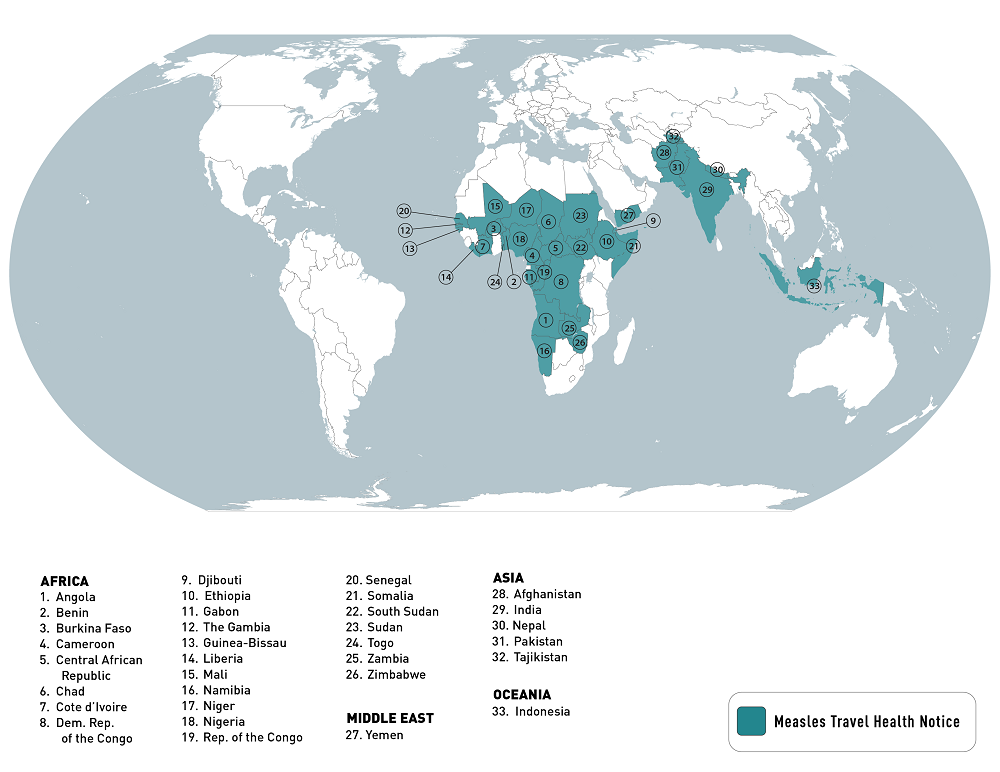
The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Watch - Level 1, Practice Usual Precautions notice regarding the ongoing worldwide measles outbreak.
As of May 16, 2023, the CDC has compiled an extensive list of countries reporting measles outbreaks in 2023.
This list is led by India, with about 68,000 measles cases.
Measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
The measles virus can live for up to two hours in airspace after an infected person leaves an area.
Furthermore, people can spread measles up to four days before and four days after a rash.
While there have only been ten measles cases in the U.S. this year, this virus remains a risk to anyone under-vaccinated.
The CDC says all international travelers, including infants and preschool-aged children, should be protected against measles before traveling abroad.
Various measles vaccines are offered in the U.S. at clinics and community pharmacies.