Search API
Icosavax, Inc. today announced positive topline interim results from its Phase 1 clinical trial of IVX-A12 against the respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) in older adults.
IVX-A12 comprises IVX-121, Icosavax’s RSV prefusion F protein VLP vaccine candidate, and IVX-241, Icosavax’s hMPV prefusion F protein VLP vaccine candidate.
“IVX-A12 is a potential first-in-class combination vaccine candidate designed to address an unmet medical need in older adults, and we believe these interim data for hMPV in an older adult population also break new ground in the field. Furthermore, as has been seen previously with prefusion F antigen approaches for RSV, we expect that a combination vaccine displaying prefusion F antigens for both hMPV and RSV, on our VLP technology, may translate into significant protection against two leading causes of pneumonia. Therefore, we plan to expeditiously proceed towards a Phase 2 trial for IVX-A12 in mid-2023 in older adults as we pursue our goal of developing a broader viral respiratory vaccine,” said Adam Simpson, Chief Executive Officer of Icosavax, in a press release on May 22, 2023.
In this Phase 1 trial, IVX-A12 induced robust immune responses against RSV and hMPV at Day 28 in older adults across dosage levels and with and without adjuvant.
When administered in combination, there was no evidence of immune interference between RSV and hMPV VLPs.
The IVX-121 (RSV) component of IVX-A12 (RSV/hMPV) previously demonstrated positive immunogenicity and tolerability results in a Phase 1/1b study, and a subset of these Phase 1b older adult subjects continue to be followed.
In December 2022, Icosavax reported positive durability data at six months, with twelve-month immunogenicity data expected in mid-2023.
Discovered in 2001, HMPV is in the Pneumoviridae family along with RSV, says the U.S. CDC.
Broader use of molecular diagnostic testing has increased the identification and awareness of HMPV as an important cause of upper and lower respiratory infections.
Other RSV vaccine candidate news is posted by Precision Vaccinations.

Why do some parents resist offering cancer prevention vaccines to their adolescents? These effective vaccines have significantly reduced cervical cancer over decades.
HPV vaccination could prevent more than 90% of cancers caused by Human Papillomavirus (HPV) from developing.
A new study published today by the American Academy of Pediatrics found parents cited vaccine safety as a leading reason for not intending to vaccinate their adolescent children against HPV has increased over time.
Overall, parental HPV vaccine hesitancy decreased by 5.5% annually between 2010 and 2012 and remained stable for nine years from 2012 through 2020.
The five most frequently cited reasons for not intending to vaccinate included “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
The proportion of parents citing “safety or side effects” as a reason for vaccine hesitancy increased significantly by 15.6% annually from 2010 to 2018.
The proportion of parents citing “not recommended,” “lack of knowledge,” or “child not sexually active” as reasons for vaccine hesitancy decreased significantly by 6.8%, 9.9%, and 5.9%, respectively, per year.
And no significant changes were observed for parents citing “not necessary.”
The good news is by 2020, about 75% of adolescents had received at least one HPV vaccine dose, but only 59% completed the vaccination series.
The U.S. CDC updated its recommended HPV vaccination schedule in 2023.
Precision Vaccinations post other sexually transmitted disease vaccine news.
According to Paraguay's National Animal Health and Quality Service President, Dr. José Carlos Martin, on May 22, 2023, three confirmed outbreaks of avian influenza (bird flu) in the Chaco region occurred, while two others were still under investigation.
MercoPress, a news agency based in Uruguay, reported bird flu was registered in home-breeding establishments with open-air sheds and backyard poultry, in Mariscal Estigarribia, Colonia Neuland, and Filadelfia, in the department of Boquerón.
On May 18, 2023, the Pan American Health Organization announced bird flu outbreaks are mainly occurring in areas along the Pacific flyway and that outbreaks have occurred in 15 countries in Latin America and the Caribbean, which it said is unprecedented.
In North America, Canada and the United States have been battling the virus since early 2022 and have reported it in wild birds, poultry, and mammals.
Additional bird flu outbreak news regarding mammals and people is posted at Precision Vaccinations.
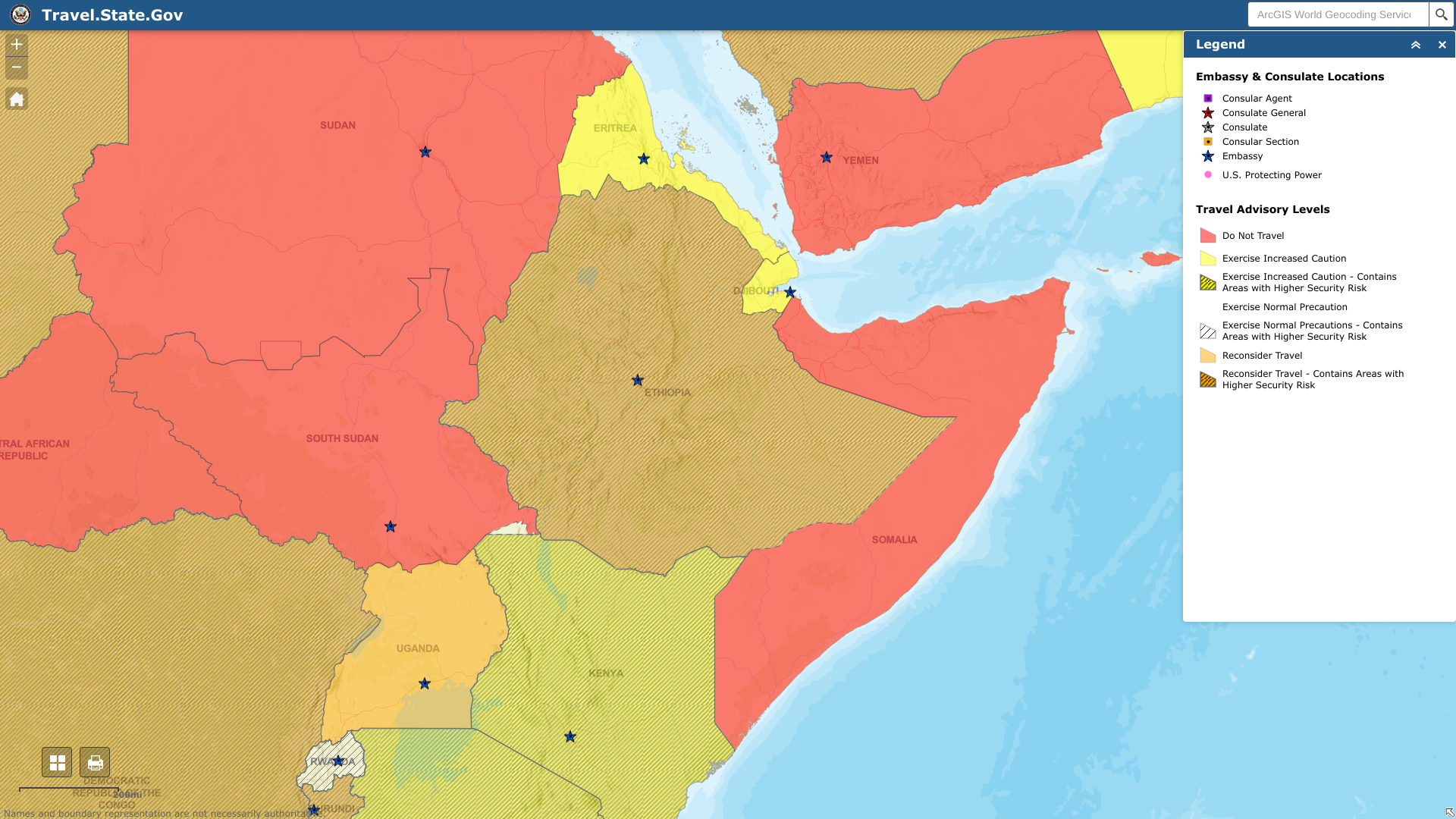
The U.S. Department of State recently reissued its Level 3 travel advisory for the Federal Democratic Republic of Ethiopia with updates to security information.
As of May 19, 2023, the U.S. says to reconsider travel to Ethiopia in cities and border areas due to sporadic civil unrest, crime, and communications disruptions.
U.S. officials have limited ability to provide services to U.S. citizens outside of Addis Ababa and any U.S. citizen detained by Ethiopian authorities.
And the government of Ethiopia has previously restricted or shut down internet, cellular data, and phone services before, during, and after civil unrest. These restrictions impede the U.S.
Please contact the Embassy’s American Citizen Services Unit at [email protected] for further assistance, and enroll in STEP to be located during emergencies.
When visiting Ethiopia, Do Not Travel To the following areas:
- Tigray Region and the border with Eritrea.
- Afar-Tigray border areas.
- Amhara Region.
- Gambella and Benishangul Gumuz Regions.
- Oromia Region.
- Southern Nations and National People Region.
- The border area with Somalia.
- Border areas with Sudan and South Sudan.
- Border areas with Kenya.
From a health perspective, the U.S. CDC suggests visitors ensure they are protected against measles and polio.
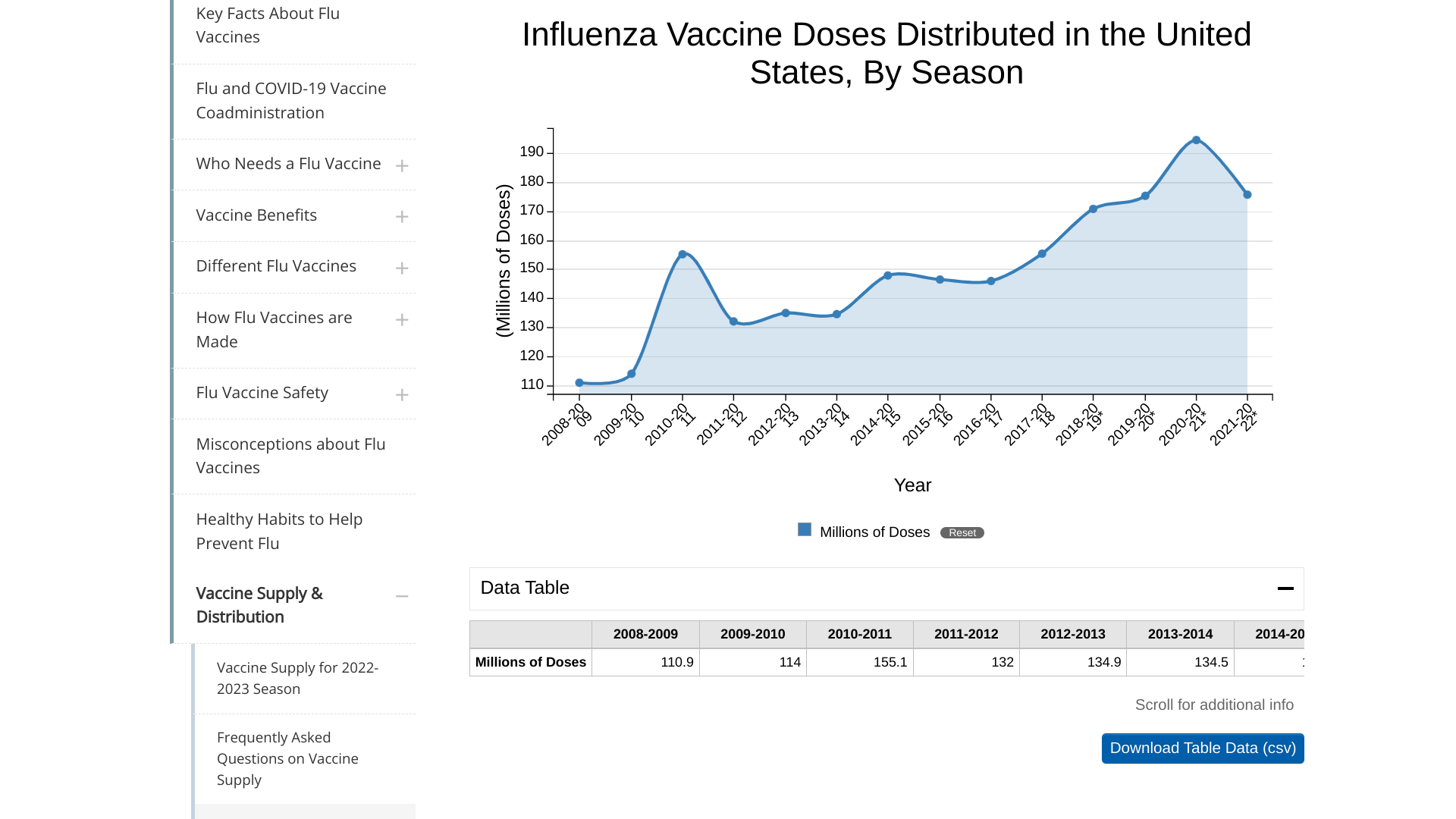
The Insight Partners today published its latest research report on "Influenza Vaccines Market Size Report, Share, Trends, Growth, Demand & Forecast to 2028, which estimated the CAGR of 7.4% from 2022 to 2028.
During the current flu season, the Centers for Disease Control and Prevention (CDC) reported as of March 4, 2023, about 173.37 million influenza vaccines had been distributed in the U.S.
This amount resembles the 2021-2022 flu season, when 174.9 million doses were distributed.
Additional flu shot news is posted by Precision Vaccinations.
Micron Biomedical recently announced positive Phase 1/2 data from the first-ever clinical trial of microarray technology in children, including infants as young as nine months old.
The study evaluated the safety, immunogenicity, and acceptability of the leading commercially available measles-rubella (MR) vaccine administered via microarray.
Vaccination by microarray was found safe and well tolerated with no allergic reactions or related serious adverse events.
Day-42 immunogenicity showed seroprotection rates for MR in all cohorts for both the microarray (93.2% - 100%) and SC injection (89.8% - 100%) groups.
And in infants who were MR-vaccine naïve at the start of the trial, seroconversion rates were high and similar for both the microarray (92.9% -100%) and SC injection groups (89.7%-100%).
Over 90% of the parents of toddlers and infants enrolled in the trial, which took part in an acceptability survey, said that the microarray technology would be better than SC injection to give vaccines to children.
"Supporting innovations in vaccine delivery is critical to addressing ongoing health inequities," said James Goodson, Senior Scientist and Epidemiologist in the Global Immunization Division at the Centers for Disease Control and Prevention and co-investigator for the study, in a press release on May 17, 2023.
The technology significantly simplifies the transport, storage, and administration of vaccines traditionally delivered via injection and eliminates sharps waste.
The U.S. CDC Advisory Committee on Immunization Practices (ACIP) confirmed it had scheduled a vaccine review meeting open to the public for June 21-23, 2023.
Conducted at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, this meeting's purpose is to review scientific data and vote on vaccines and candidate recommendations for Respiratory Syncytial Virus vaccines; Recommendations for adult Polio vaccinations; Flu shots for the 2023-2024 season, Chikungunya, COVID-19, Dengue, Meningococcal, and Mpox vaccines.
This meeting's agenda will be led by Dr. Grace Lee, the ACIP Chair.
ACIP recommendations are public health guidance for the safe use of vaccines and related biological products.
The non-binding recommendations include the age(s) when the vaccine should be given, the number of doses needed, the amount of time between doses, and precautions and contraindications.

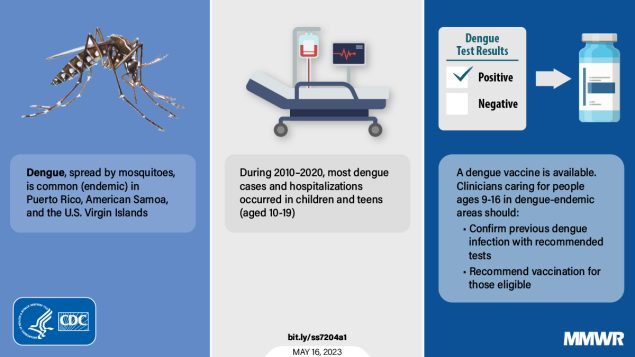
Dengue is one of the most common vectorborne flaviviral infections globally, with frequent outbreaks in the tropical regions of the United States, says the U.S. Centers for Disease Control and Prevention (CDC).
The CDC confirmed on May 19, 2023, U.S. territories experienced a high prevalence of dengue during 2010–2020, a total of 30,903 dengue cases were reported from four U.S. territories.
Puerto Rico reported the highest number of dengue cases (29,862).
And in Puerto Rico and USVI, approximately 2% of reported cases were categorized as severe dengue.
The Florida Health Department reported on May 13, 2023, there had been 68 travel-associated dengue cases and two locally acquired dengue cases confirmed as of week #17 in 2023. And Miami-Dade County remains under a mosquito-borne illness alert.
Floria reported 903 travel-associated and 68 locally-acquired dengue cases, the most noted in the U.S. in 2022.
Dengue is a vaccine-preventable disease, with two vaccines in use globally.
In addition, travel disease hot-spot news is posted by Vax-Before-Travel.