Search API
The U.S. Centers for Disease Control and Prevention (CDC) recently reported 152 of 2,144 (7.09%) people were sickened with norovirus during the Celebrity Cruises Summit cruise to Bermuda between May 15–May 25, 2023.
Twenty-five crew members also reported being ill during the voyage.
Norovirus is a very contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus, says the CDC.
As of May 31, 2023, Celebrity Cruises reported the following actions:
- Increased cleaning and disinfection procedures according to the ship’s outbreak prevention and response plan.
- Notified current guests of the situation onboard and encouraged illness reporting and good hand hygiene.
- Collected and sent stool specimens from gastrointestinal illness cases to the CDC laboratory.
- Provided twice daily reports of gastrointestinal illness cases to VSP during the outbreak investigation.
- Consulted with VSP about sanitation cleaning procedures and reporting ill cases.
- Notified embarking guests of the situation onboard and encouraged illness reporting and good hand hygiene.
Throughout 2023, the CDC has reported ten other norovirus outbreaks on cruise ships.
As of June 7, 2023, there are norovirus vaccine candidates conducting clinical studies.
GSK plc today announced that the European Commission had authorized Arexvy™ for active immunization to prevent lower respiratory tract disease (LRTD) caused by a respiratory syncytial virus (RSV) in adults 60 and older.
The first launches are planned for the 2023/2024 RSV season, which typically starts in the autumn.
Tony Wood, Chief Scientific Officer, GSK, commented in a press release on June 7, 2023, "This authorization for Arexvy means eligible adults can be vaccinated against RSV disease for the first time, reinforcing GSK's long history of vaccine innovation."
Our strong manufacturing capability and scale, including from our vaccine manufacturing site in Belgium, means we are ready to deliver the (RSV) vaccine as countries begin to launch."
RSV is a common, contagious respiratory virus that leads to over 270,000 hospitalizations and approximately 20,000 in-hospital deaths each year in adults aged 60 years and over in Europe.
Those with underlying medical conditions, such as diabetes and chronic heart and lung disease, drive the majority of RSV hospitalizations.
In the U.S., two RSV vaccines have been authorized for seniors, and one RSV monoclonal antibody (Beyfortus®) is seeking authorization for infants/children.

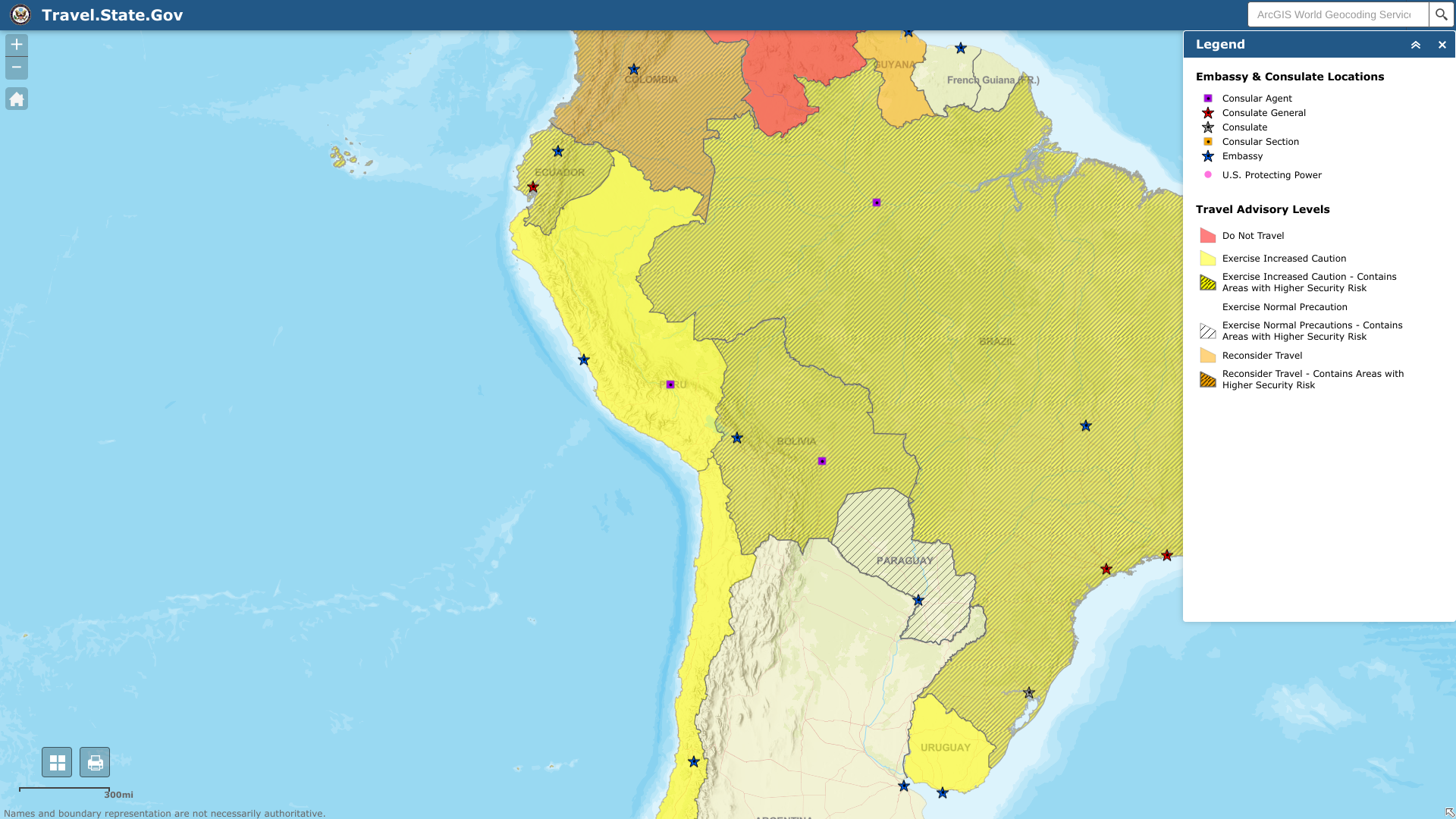
The U.S. Department of State today reissued Level 2: Exercise Increased Caution travel advisories for two additional South American countries, the Plurinational State of Bolivia and the Republic of Ecuador.
On June 6, 2023, the State Department confirmed both countries are confronting civil unrest.
Previously, on June 1, 2023, the State Department issued a similar advisory for Peru.
If you decide to visit these countries, monitor local media for breaking events, avoid demonstrations, and be prepared to adjust your plans, says the U.S. government.
And prepare a contingency plan for emergencies and enroll in the Smart Traveler Program to be located in a crisis.
From a health perspective, the U.S. CDC suggests speaking with a travel vaccine provider about one month before visiting these countries to review immunization and medication options targeting diseases such as dengue and yellow fever.
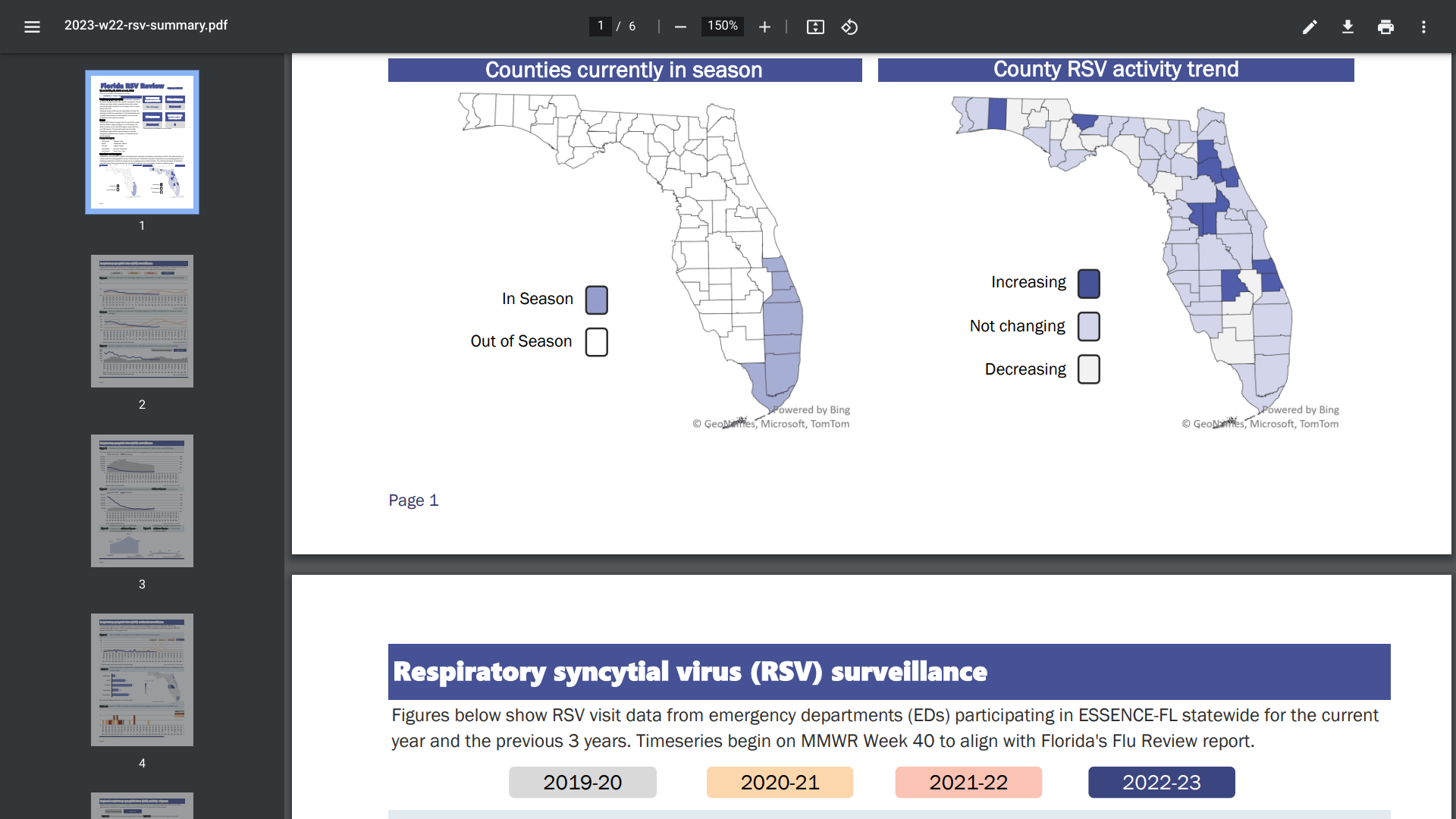
The Florida Department of Health published an updated Respiratory Syncytial Virus (RSV) weekly report that indicates an increased positivity rate but no outbreaks as of June 3, 2023.
This is positive news since Florida’s RSV season is traditionally more extended than the rest of the U.S.
During the summer of 2021, Florida reported an unseasonably high number of RSV outbreaks, but activity during the 2021-22 season returned to a more typical trend.
From a global perspective, the World Health Organization Influenza Update N° 446, published on May 29, 2023, disclosed RSV activity was generally low except in Australia and a few countries in the Region of the Americas.
And RSV has increased in several countries in tropical and temperate South America.
Furthermore, the U.S. is better prepared for the 2023-2024 RSV season than ever.
As of June 6, 2023, there are two approved RSV vaccines and several candidates conducting late-stage studies.
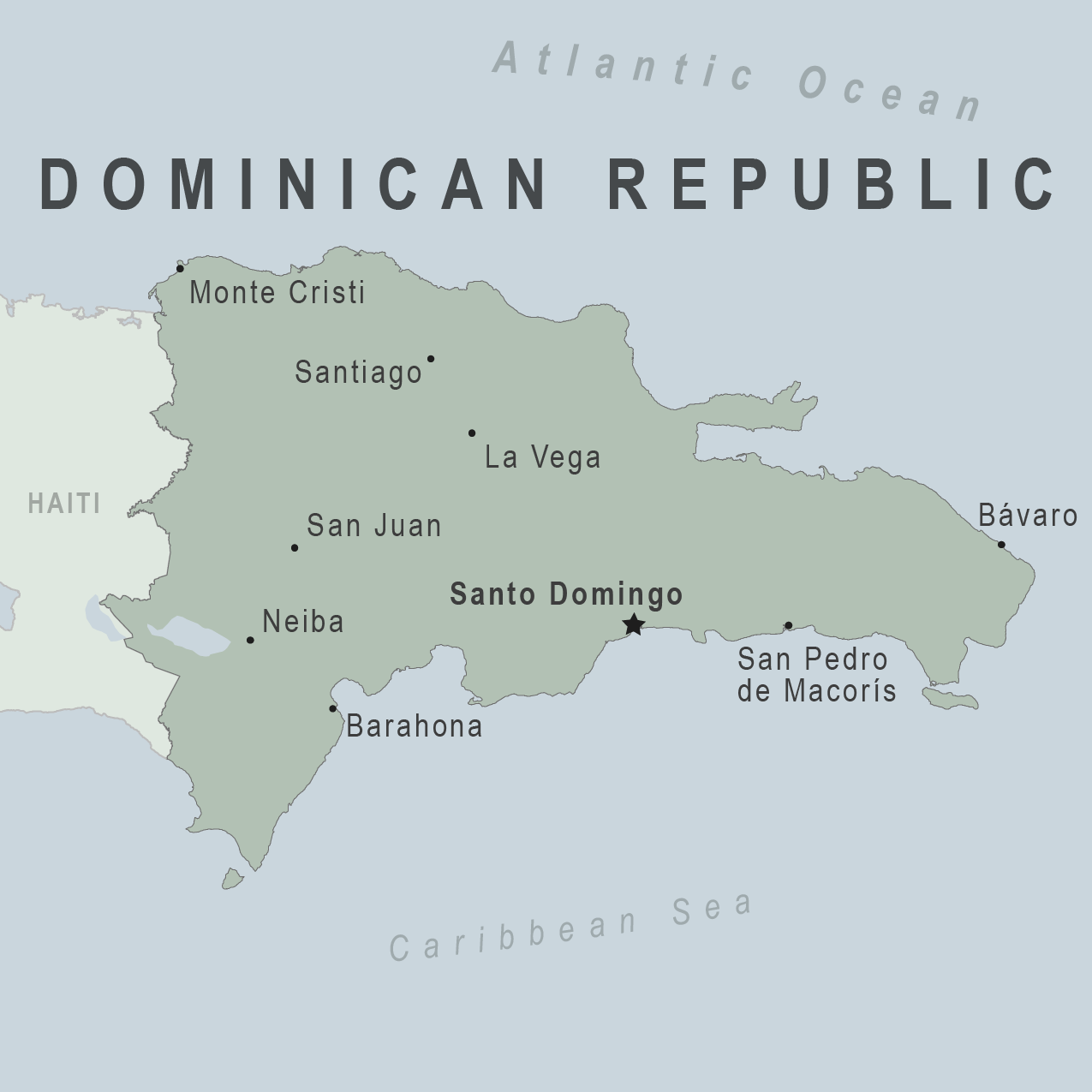
The U.S. Department of State today reissued its Level 2: Exercise Increased Caution for the Dominican Republic, which is located on the island of Hispaniola in the Caribbean region.
On June 6, 2023, the State Department confirmed civil unrest and health issues remain a concern for visitors.
The development of a professional tourist police corps, the institution of a 911 system in many parts of the country, and a concentration of resources in resort areas mean these tend to be better policed than urban areas like Santo Domingo.
From a health perspective, Dengue is a risk in many parts of the Caribbean, including the Dominican Republic.
Furthermore, check your vaccines and medicines and visit a healthcare provider at least a month before your trip to get what you need while traveling, says the U.S. CDC.
The journal Vaccine published results from a peer-reviewed study that concluded a fourth dose of Novavax Inc.'s NVX-CoV2373 protein-based COVID-19 vaccine enhanced immunogenicity for ancestral and variant SARS-CoV-2 strains without increasing reactogenicity, indicating that updates to the vaccine composition may not be currently warranted.
Furthermore, correlates of protection imply post-boost efficacy of ≥ 82% for Omicron variants.
Published on June 2, 2023, this study reported a fourth dose of NVX-CoV2373 (5 µg SARS-CoV-2 recombinant spike protein + 50 µg Matrix-M™ adjuvant) did not increase local/systemic reactogenicity, enhanced immune response to SARS-CoV-2 variants, and NVX-CoV2373 fourth dose induced robust immunogenicity in those aged 18–84 years.
In conclusion, despite the call for variant-specific vaccines, an increase in the number of vaccine booster doses with NVX-CoV2373 enhances immunogenicity for the ancestral SARS-CoV-2 strain and its variants without a notable increase in reactogenicity. Therefore, these data suggest that further boosting with the ancestral sequence used in NVX-CoV2373 should retain meaningful utility in preventing variant virus-associated illness, wrote these researchers.
This work was supported by Novavax, Inc. and initially by the Coalition for Epidemic Preparedness Innovations.
As of June 5, 2023, Novavax COVID-19 Vaccine (Nuvaxovid, CovoVax, NVX-CoV2373) was authorized in forty markets.




