Search API
Bavarian Nordic A/S today announced today the initial safety and immunogenicity results from a randomized, double-blind, placebo-controlled Phase 3 clinical trial of a virus-like particle (VLP)-based chikungunya virus (CHIKV) vaccine candidate CHIKV VLP (PXVX0317) in healthy adults.
The initial results up to Day 22 post-vaccination showed that CHIKV VLP was immunogenic in healthy adults ≥65 years of age, as demonstrated by a strong induction of CHIKV neutralizing antibodies in 87% of vaccinees with neutralizing antibody titres exceeding the threshold agreed with authorities as a marker of seroprotection, thus meeting the primary endpoints of the study.
Importantly, seroprotective neutralizing antibodies were also observed in most subjects (82%) at Day 15 post the single vaccination, demonstrating a fast onset of protection for the VLP-based CHIKV vaccine candidate.
This clinical trial will continue for a 6-month follow-up for both safety and immunogenicity.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on June 20, 2023, “While we still await the results from the second and larger Phase 3 study in adolescents and adults later this year, these highly encouraging results provide a high degree of confidence for our CHIKV vaccine program.”
Chikungunya is a mosquito-borne viral disease caused by the Chikungunya virus (CHIKV), causing outbreaks in over 100 countries as of 2023.
From 2006–2021, 4,590 chikungunya cases in U.S. travelers were reported to the U.S. CDC.
While mortality is low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Additional Chikungunya vaccine news is posted by Precision Vaccinations.
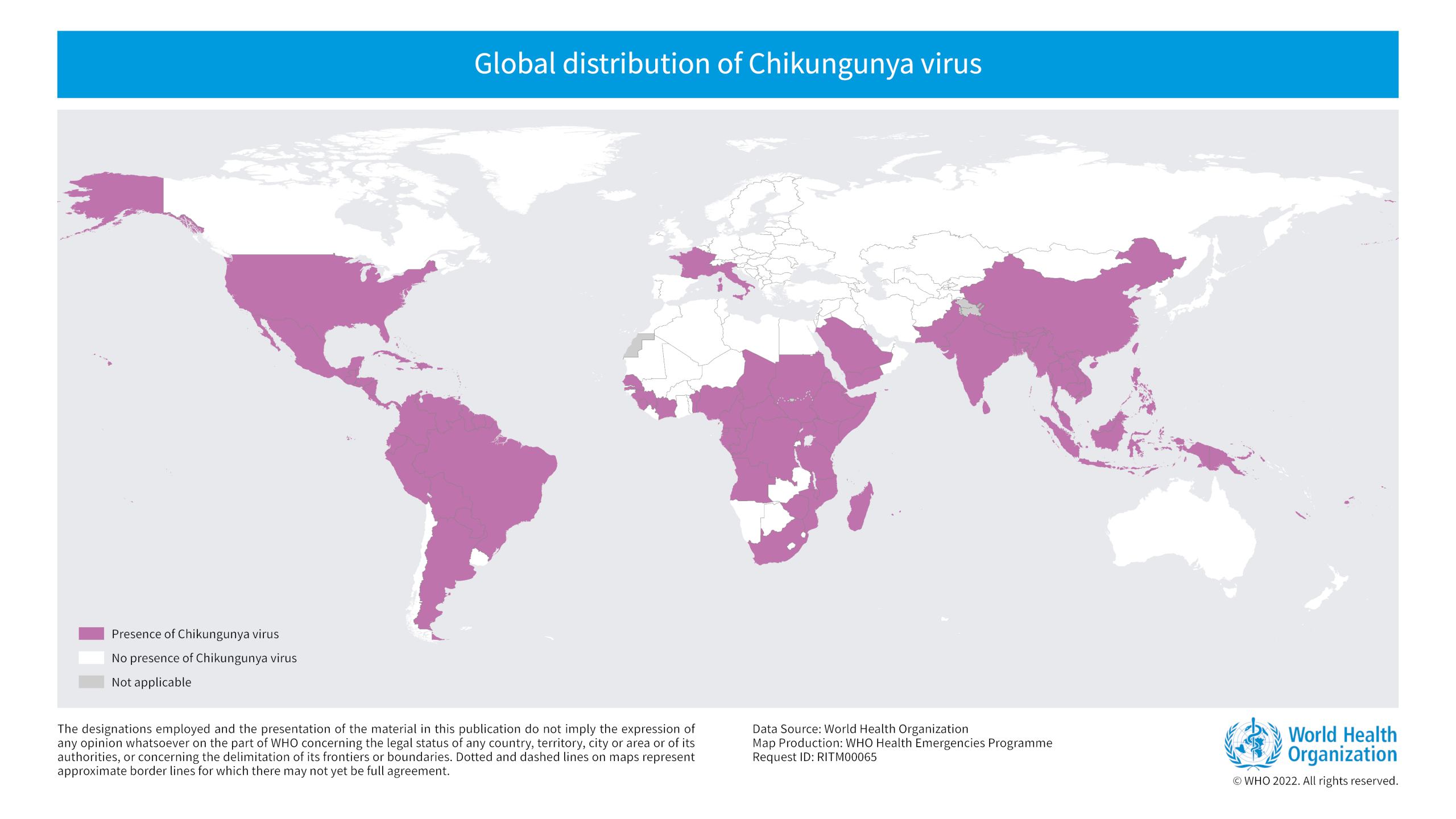
The European Centre for Disease Prevention and Control (ECDC) recently confirmed sporadic human cases of avian influenza A (H9N2) have been observed in 2023, mainly in young children.
As of June 12, 2023, one new human infection with H9N2 was reported in Sichuan province, China.
And since 1998, a total of 125 laboratory-confirmed cases, including two deaths, of human infection with H9N2 viruses have been reported in eight countries: China (112), Egypt (4), Bangladesh (3), Cambodia (2), Oman (1), Pakistan (1), India (1), and Senegal (1).
Over the past year, various humans have been infected with different avian influenza viruses.
According to the Centers for Disease Control and Prevention, Avian influenza or bird flu is caused by infection with avian (bird) influenza (flu) Type A viruses.
And the Highly Pathogenic Avian Influenza (HPAI) strain of the H5N avian flu is currently spreading in the U.S.
In the U.S., the government has already authorized one bird-flu vaccine and invested in developing other vaccine candidates.
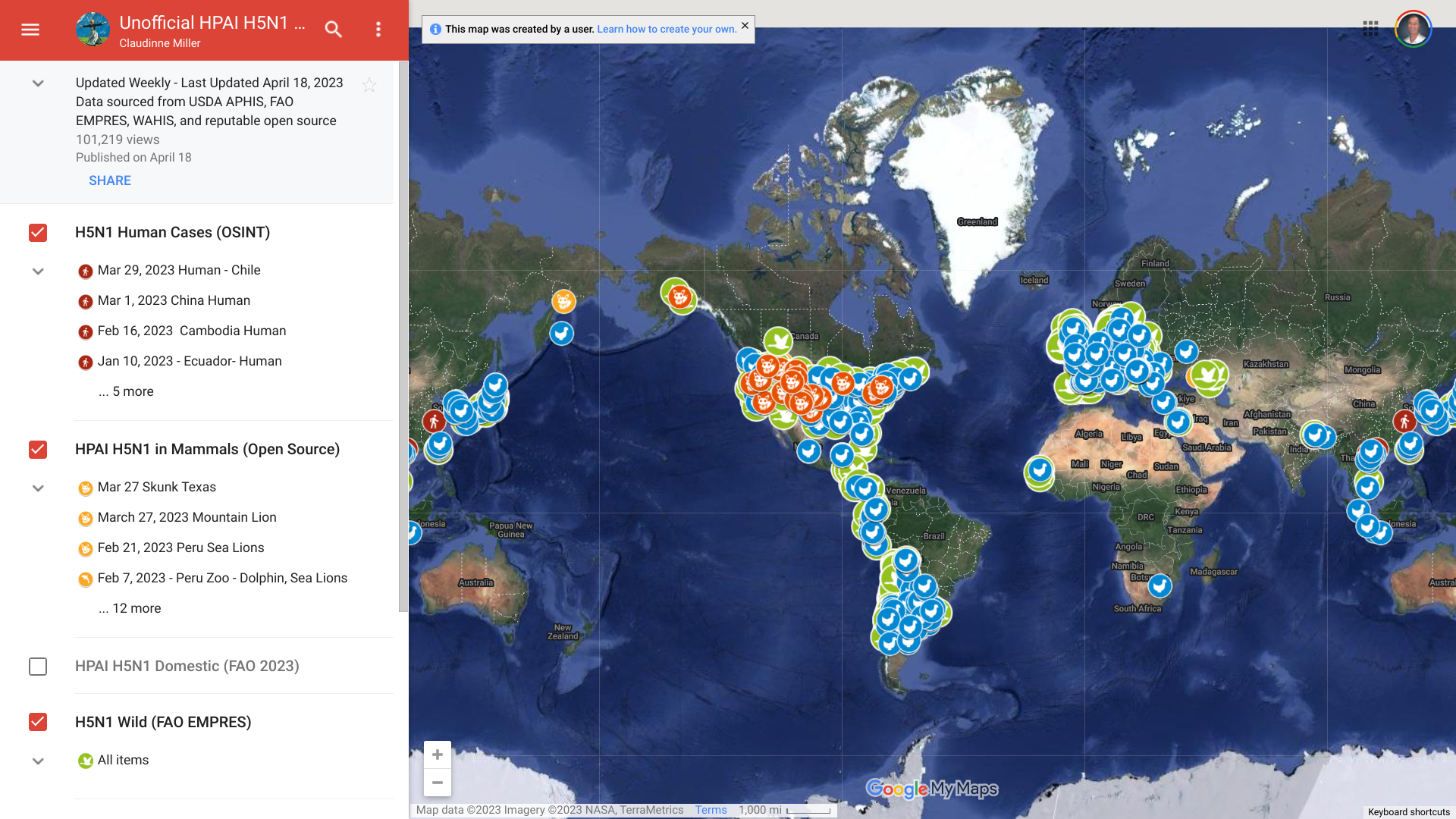
The European Centre for Disease Prevention and Control (ECDC) recently announced that since the beginning of 2023, about 242 measles cases have been reported by 12 European countries.
Austria reported 121 cases of measles in 2023.
According to national data, as of June 13, 2023, Styria is the most affected region in Austria, with 102 cases reported since the beginning of the outbreak in early 2023.
Measles outbreaks have also been reported from other regions, including Upper Austria (5), Lower Austria (4), Vienna (5), Carinthia (4), and Burgenland (1).
As of June 14, 2023, surveillance sources had detected 22 new suspected and/or confirmed measles cases reported in seven EU/EEA countries over the past month: Estonia (2), Germany (12), Ireland (2), Poland (2), Spain (1), and Sweden (1).
So far, in 2023, one measles-related death has been reported in the EU/EEA (the Netherlands).
And the U.S. CDC reported on June 12, 2023, India has confirmed 73,536 measles cases over the past year.
In the U.S., a total of 16 measles cases were reported by 11 jurisdictions as of early June 2023.
Measles is a very contagious disease that is preventable with various vaccines.
SK bioscience today announced the company has received the WHO Emergency Use Listing (EUL) of its COVID-19 vaccine, SKYCovione™ (SKYCovion™, GBP510).
This COVID-19 vaccine was developed with the Institute for Protein Design (IPD) at the University of Washington SCHOOL OF MEDICINE and uses GSK's pandemic adjuvant.
SKYCovione is also the world's first medicine developed using computational protein design, an approach that uses Rosetta software to engineer protein structures with enough precision to place individual atoms exactly where desired.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release on June 19, 2023, "Based on the immunogenicity and safety profile, SKYCovione has become the first Korean vaccine to be granted to the WHO EUL."
"We will be committed to developing more vaccines not just to strengthen Korea's vaccine sovereignty but also to enable equitable access to the vaccine."
The development of SKYCovione has been supported by funding from the Bill & Melinda Gates Foundation and Coalition for Epidemic Preparedness Innovations with support from the European Horizon 2020 Programme.
SKYCovione (known as SKYCovion in the UK) was approved by the Medicines and Healthcare products Regulatory Agency for adults in May 2023.
SKYCovione is a self-assembled nanoparticle vaccine targeting the receptor binding domain of the SARS-CoV-2 Spike protein for SARS-CoV-2.
The vaccine can be stored between 2-8 °C, making it suitable for use in countries where ultra-low cold chain storage facilities are unavailable. The ease of distribution helps to achieve greater access to vaccines in low-income countries.
Other COVID-19 vaccine news is posted by Precision Vaccinations.

International visitors may be exposed to several infectious diseases in the Republic of Costa Rica in 2023, reported the U.S. Centers for Disease Control and Prevention (CDC).
As of June 18, 2023, though malaria cases have been reported primarily in the Caribbean province of Limon, there is a risk of contracting this mosquito-transmitted disease anywhere in Costa Rica, says the local U.S. embassy.
Costa Rica has been advancing various programs toward the elimination of malaria by the year 2025.
Additionally, Costa Rica has been suffering an outbreak of dengue (both classic and hemorrhagic) for a few years.
As of epidemiological week #16 in 2023, a total of 1,179 cases of dengue were reported, with most of the dengue cases found in the Huetar Caribe and Central Sur regions.
Dengue is a vaccine-preventable disease, with two vaccines currently authorized in various countries.
And from a security perspective, the U.S. Embassy in San Jose recently alerted U.S. citizens of increasing civil unrest in San Jose and suggests enrolling in the Smart Traveler Program to receive emergency notifications.

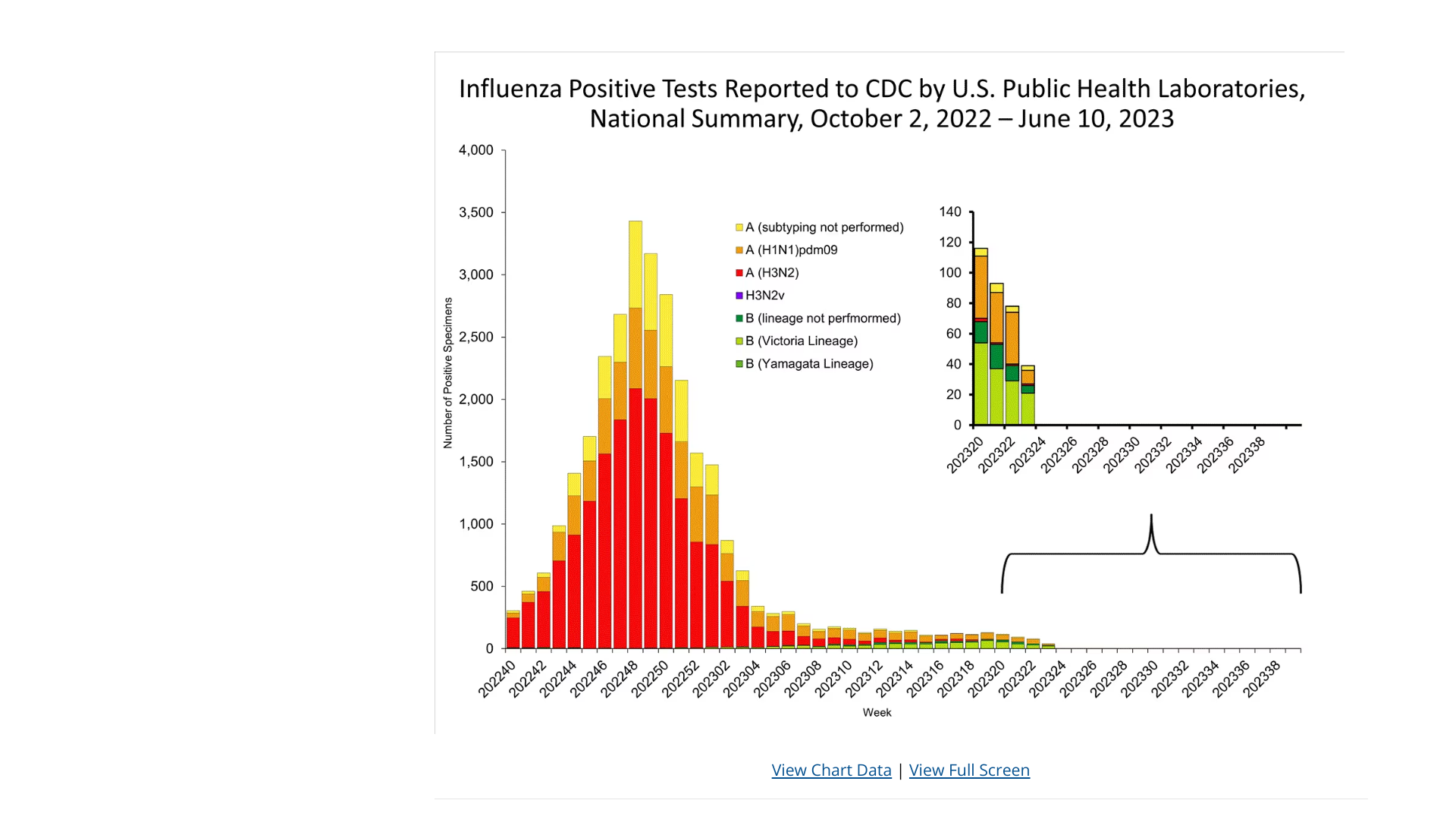
The U.S. Centers for Disease Control and Prevention (CDC) Weekly U.S. Influenza Surveillance Report indicated minimal flu activity in the U.S.
Based on the National Center for Health Statistics Mortality Surveillance data available on June 15, 2023, 6.3% of the deaths during week #23 were due to pneumonia, influenza, and/or COVID-19 (PIC).
Among the 1,295 PIC deaths reported for last week, ten listed influenza.
Furthermore, in the southern hemisphere, Australia's Department of Health and Aged Care published report No. 4, which stated there is not enough information to assess the potential severity of the 2023 influenza season.
Since seasonal surveillance commenced in Australia in April 2023, there have been only 518 sentinel hospital admissions with confirmed influenza as of June 2, 2023.
As of June 18, 2023, various flu shots are available worldwide.
The World Health Organization (WHO) recently reported nearly 1.5 million new COVID-19 cases and 7,300 related fatalities in the last 28 days (May 15 to June 11, 2023).
As of June 15, 2023, all six WHO regions reported (Edition #147) decreases in COVID-19 cases and fatalities.
During the current 28-day period, only 59% of countries and territories reported cases, a proportion that has been declining since mid-2022.
Various COVID-19 vaccines and antibody therapies remain available in most countries as of June 18, 2023.