Search API
The U.S. Centers for Disease Control and Prevention (CDC) today published a Health Alert Network (CDCHAN-00495) reminding healthcare professionals seeing patients affected by wildfire smoke to be alert to the possible adverse effects, particularly among individuals at higher risk of severe outcomes.
Wildfire smoke exposure may exacerbate respiratory, metabolic, and cardiovascular chronic conditions like asthma, chronic obstructive pulmonary disease, and congestive heart failure.
Furthermore, people can be impacted even if they are not near the fire source due to exposure to particles of PM2.5, which are inhalable air pollutants with aerodynamic diameter ≤2.5 microns.
The acute signs and symptoms of smoke exposure include headache, eye, and mucous membrane irritation, dyspnea (trouble breathing), cough, wheezing, chest pain, palpitations, and fatigue.
The CDC suggests people in effected areas stay indoors and limit time outdoors. If you must go outside when smoke is visible or can be smelled, reduce your smoke exposure by wearing an N95 or P100 respirator.
And keep track of smoke near you using AirNow’s “Fire and Smoke Map” or the AirNow app or by listening to the Emergency Alert System and National Oceanic and Atmospheric Administration Weather Radio.

Vaxxinity, Inc. recently announced positive results from Part B of its Phase 1 clinical trial of UB-312, an investigational vaccine for Parkinson's disease (PD).
On June 22, 2023, the Company confirmed in a press release that UB-312 was well-tolerated and induced anti-alpha-synuclein (aSyn) antibody responses in participants with early PD, meeting the trial's primary objectives.
UB-312 is an investigational synthetic peptide vaccine targeting toxic aggregated aSyn forms to address PD and other synucleinopathies. Alpha-synuclein plays a central role in synaptic functions and regulation of neurotransmitter release.
The accumulation and aggregation of misfolded aSyn in the brain are key factors in PD's development and progression.
"This positive Phase 1 results demonstrate several important features necessary for an immunotherapy against Parkinson's disease and other synucleinopathies to be successful and represent a further proof-of-principle for Vaxxinity's platform in chronic disease," said Mei Mei Hu, CEO of Vaxxinity.
"UB-312 was observed to safely break immune tolerance, inducing antibodies against toxic aggregated forms of alpha-synuclein."
"Importantly, these antibodies crossed the blood-brain barrier, and the data also suggest potential target engagement in the periphery, where pathological alpha-synuclein is known to be concentrated."
"Together, these results support the further development of UB-312 in a Phase 2 clinical trial."
"We continue to view UB-312 as a promising candidate for the prevention or disease modification of Parkinson's disease globally."
This announcement is important since PD affects approximately one million people in the U.S. and more than 10 million worldwide.
The U.S. NIH says PD is a chronic brain and progressive neurodegenerative disorder that affects predominately dopamine-producing neurons in the substantia nigra area of the brain.
Additionally, The Michael J. Fox Foundation is funding a 2-year collaborative project between Vaxxinity, the Mayo Clinic, and the University of Texas Houston. This work evaluates the potential of protein misfolding cyclic amplification to assess target engagement and will also aim to characterize the anti-aSyn antibodies produced after UB-312 administration.

SK bioscience today announced positive results from its Phase II clinical trials in infants of its 21-valent pneumococcal conjugate vaccine candidate, 'GBP410' (SP0202), evaluating its safety and immunogenicity.
Given that GBP410 includes 21 serotypes, it is anticipated to offer broader serotype coverage than the existing pneumococcal conjugate vaccines.
The Phase II study demonstrated comparable immunogenicity of GBP410 compared to the control vaccine, following the primary vaccination at 2, 4, and 6 months of age as well as the booster vaccination for ages of 12 to 15 months.
The data also showed a well-tolerated safety profile, with a similar reactogenicity profile to the control vaccine and no vaccine-related serious adverse events.
Furthermore, GBP410 did not interfere with the immunogenicity and safety profile of the co-administered recommended pediatric vaccines, such as tetanus, diphtheria, pertussis, polio, and Haemophilus influenzae type b vaccines.
Based on the positive safety and immunogenicity data from the Phase II clinical trial, SK bioscience and its development partner Sanofi plan to start Phase III in H1 2024, expecting to secure the final data in 2027.
In preparation for the commercialization of GBP410, SK bioscience intends to enter the U.S. and European markets with Sanofi by making significant investments in manufacturing facilities.
Jean-Francois Toussaint, Global Head of Vaccines R&D at Sanofi, said in a press release on June 29, 2023, "We are pleased with our very productive partnership with SK bioscience as we work to raise the bar in pneumococcal disease."
"With an innovative carrier that breaks the glass ceiling of serotype compositions, our 21-valent pneumococcal conjugate vaccine is designed to offer expanded protection against this devastating disease."
"We believe that today's results offer us a strong path to Phase 3 and then to licensure."

As the new flu season approaches this fall, influenza viruses from last season continue negatively impacting people.
On June 30, 2023, the U.S. Centers for Disease Control and Prevention (CDC) published the Weekly U.S. Influenza Surveillance Report confirmed respiratory illness, often called influenza-like illness, severely impacts people.
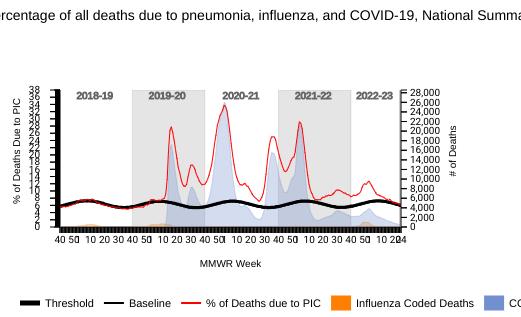
According to National Center for Health Statistics Mortality Surveillance data on June 29, 2023, 6.2% of the deaths during week #25 were due to pneumonia, influenza, and/or COVID-19 (PIC).
Among the 1,342 PIC deaths reported for this week, 240 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, and eight listed as influenza.
The majority of deaths were related to pneumonia.
Furthermore, one additional influenza-associated pediatric death occurring during the 2022-2023 season was reported to CDC during week 25.
Throughout the 2022-2023 flu season, the total number of pediatric deaths totaled 160.
In the 2019-2020 flu season, 199 children died from influenza infections.
With the new flu season starting soon, the CDC recently announced good news regarding vaccines.
The U.S. CDC adopted the 2023-2024 recommendations on annual influenza vaccination for everyone six months and older on June 27, 2023.
Additionally, flu vaccinations in July and August are not recommended for most people, but there are several considerations for specific groups.
The U.S. Centers for Disease Control and Prevention (CDC) recently stated dengue is an ongoing risk in many parts of Asia and the Pacific Islands.
On June 28, 2023, the CDC reissued a Level 1 - Practice Usual Precautions, Travel Health Advisory that revealed the nine countries reported higher-than-usual dengue cases.
And travelers visiting these countries may be at increased risk.
Dengue is a vaccine-preventable disease caused by a virus spread through mosquito bites. The disease can take up to two weeks to develop, with illness generally lasting less than a week.
The CDC says severe health effects include bleeding, shock, organ failure, and death.
As of June 30, 2023, dengue outbreaks have been reported in Florida, Mexico, Costa Rica, and various Central and South American countries.

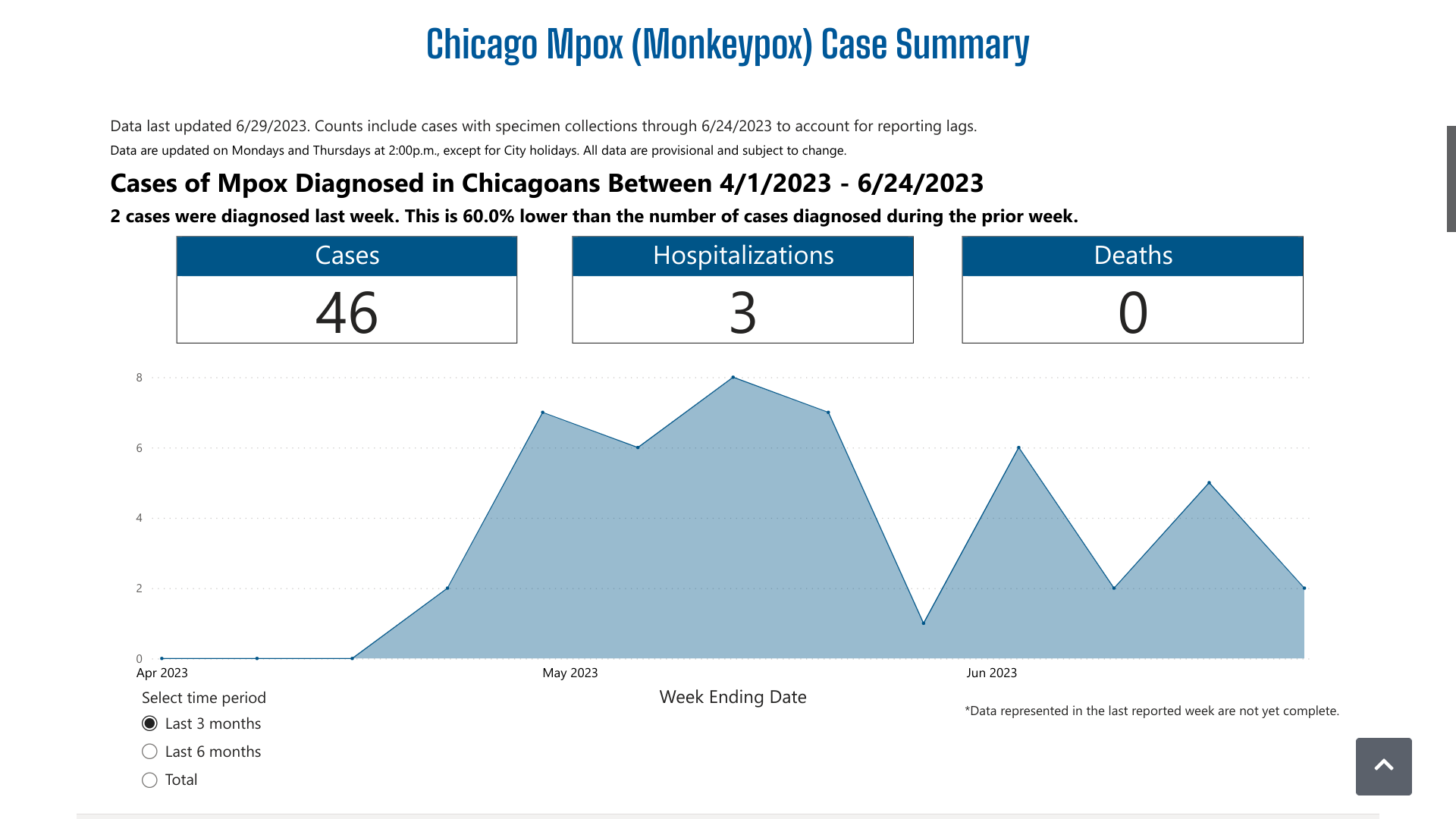
The Chicago Department of Public Health (CDPH) reconfirmed on June 27, 2023, it is investigating a recent increase in mpox cases among Chicago residents.
Between June 4 and June 22, 2023, there were six additional mpox cases reported.
The CDPH's dashboard indicates over the past three months, there have been 46 mpox cases and three related hospitalizations. And Chicago did not disclose the number of vaccine-breakthrough cases reported.
From a prevention perspective, 146 people were vaccinated in Chicago last week with the JYNNEOS® (MVA-BN) vaccine.
Since May 2022, 49,351 doses have been administered.
Chicagions with mpox questions can contact the HIV/STI Resource Hub at 844-482-4040 or the CDPH Call Center at 312-746-4835.
Throughout Illinois, including CDPH's data, 1,488 mpox cases have been reported during the global outbreak.
As of June 28, 2023, the U.S. CDC reported 30,531 mpox cases and 43 related fatalities since May 2022.
While Bavarian Nordic's vaccine has been the primary mpox vaccine offered in the U.S., a Cincinnati-based firm Blue Water Biotech, Inc., announced on June 28, 2023, preliminary preclinical data supporting the use of its norovirus shell and protrusion virus-like particle platform to develop a novel mpox vaccine candidate.