Search API
The Lancet Infectious Diseases recently published results from a phase 1 clinical trial of a Lyme disease vaccine candidate, showing that Valneva's VLA15 produces a strong but waning immune response against six common strains of the Borrelia burgdorferi bacterium found in Europe and the United States.
Valneva Austria researchers led this company-sponsored, partially randomized, observer-masked study of the novel, multivalent outer surface protein A (OspA) subunit vaccine candidate.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick.
VLA15 produced immune responses for all strains, but responses were greater in the higher-dose adjuvanted groups. And one month after the third dose, responses declined, reaching baseline by one year.
And a booster dose given 13 months after the first dose triggered a strong immune response for about six months.
This study's findings are good news since Lyme borreliosis is the most common tick-borne disease in the northern hemisphere.
In Europe, there are estimated to be more than 200,000 cases each year. And in the U.S., approximately 30,000 patients per year.
In a related commentary also published by The Lancet, Nicole Bézay, and colleagues reported Valneva's novel vaccine represents a milestone in our fight against Lyme disease."
Initially developed by France-based Valneva SE, New York-based Pfizer, Inc. is VLA15's current development and commercialization collaboration.
Pfizer previously indicated it could submit a Biologics License Application in 2025 and Marketing Authorization Application in Europe in 2026, subject to positive data.

Local media today reported Moderna Inc. intends to establish its Chinese headquarters in Shanghai to promote the research, development, production, and sales of messenger RNA vaccines and drugs in China.
Yicai Global confirmed on July 6, 2023, Moderna signed a memorandum of understanding and a land deal with Shanghai's Minhang district government, marking the Massachusetts-based firm's first investment in China.
Sources told Yicai Global that Moderna's investment will total USD1 billion.
Additionally, Moderna will build a plant in China to make respiratory disease vaccines, such as those against respiratory syncytial virus (RSV) and influenza.
As the recent global pandemic fades, there is market pressure on Moderna to find new vaccine products for its foraying into China, wrote Yicai.

The World Health Organization (WHO) today announced that 12 African countries will receive about 18 million doses of an approved malaria vaccine over the next two years.
On July 5, 2023, the WHO confirmed GSK's Mosquirix™ RTS,S/AS01 malaria vaccine will continue in Ghana, Kenya, and Malawi.
The first vaccine doses are expected to arrive in countries during the last quarter of 2023, with countries starting to roll them out by early 2024.
The Mosquirix vaccine has been administered to over 1.7 million children since 2019. It has been shown to be safe and effective, resulting in a substantial reduction in severe malaria and a fall in child deaths.
Mosquirix allocations were also made for new vaccine introductions in Benin, Burkina Faso, Burundi, Cameroon, the Democratic Republic of the Congo, Liberia, Niger, Sierra Leone, and Uganda.
These allocations were determined by applying the principles outlined in the Framework for allocating limited malaria vaccine supply.
Malaria remains one of Africa's deadliest diseases, killing nearly half a million children under five and accounting for approximately 95% of global malaria cases in 2021.
A second malaria vaccine, R21/Matrix-M™, developed by Oxford University and manufactured by the Serum Institute of India, could also be prequalified by WHO soon.
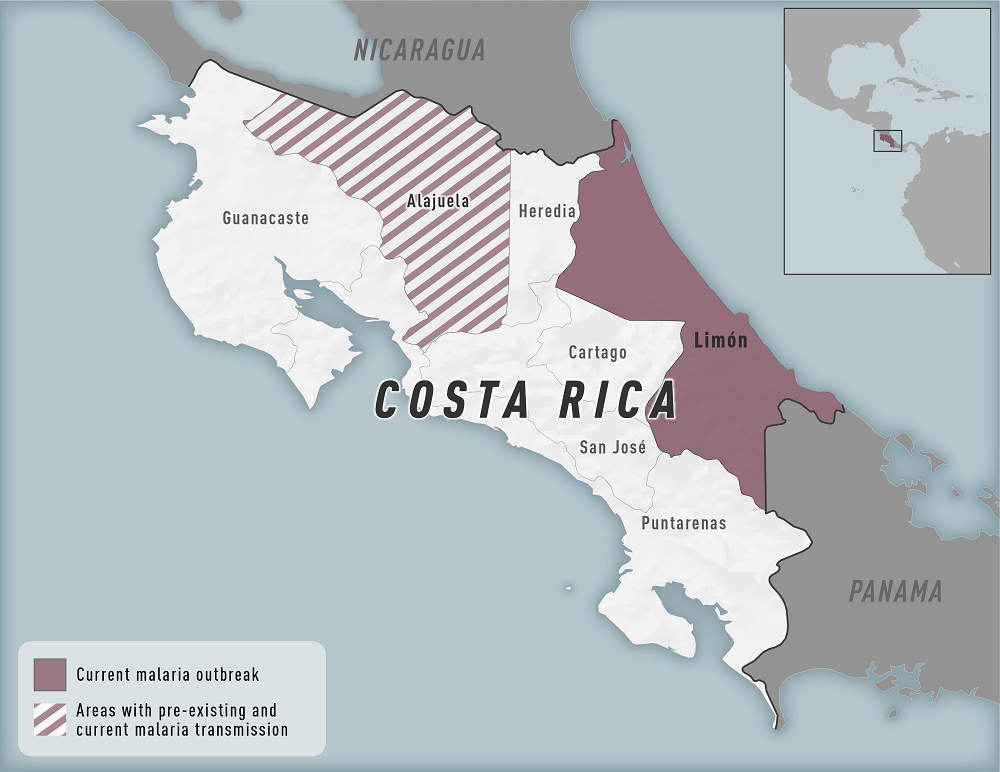
As of July 5, 2023, these malaria vaccines are not offered in the U.S. (Florida) or Costa Rica.

The European Commission (EC) recently confirmed it will facilitate the uptake of the EU Digital COVID certificate by the World Health Organization (WHO) and contribute to its operation and further development.
The EU Digital COVID certificate has been issued to over 2.3 billion people.
This means about fifty-one countries can begin accessing the WHO's Global Digital Health Certification Network (GDHCN) on July 1, 2023.
This global verification of health document system is designed to deliver better health for all.
The GDHCN's digital capabilities may include verification of vaccinations and prescriptions across country borders, verification of vaccination certificates within borders, the International Patient Summary, and certification of public health professionals through the WHO Academy.
And these capabilities include using EU privacy standards and validating digital signatures to prevent fraud.
However, the WHO will not have access to any underlying personal data, which would continue to be the exclusive domain of national governments.
This digital infrasctrure was initially presented on January 27, 2021, and confirmed in June 2023.
As of July 5, 2023, the U.S. government has not announced its official participation in the GDHCN.

U.S. Senators Tammy Baldwin and Thom Tillis introduced the Disease X Act of 2023 to develop the necessary medical countermeasures (MCMs) to combat future pandemics.
The legislation filed on June 28, 2023, provides $40 million per year for five years specifically for Disease X MCMs and requires products developed using funding from the Disease X Program.
Even though deadly infectious disease epidemics can dramatically affect the American public, military personnel, and our economic and national security, there is currently no sustained funding, program, or strategy dedicated to accelerating the development of medical countermeasures for previously identified infectious disease threats with the greatest pandemic potential, referred to as Disease X, wrote these senators.
"The Disease X Act of 2023 empowers Biomedical Advanced Research and Development Authority (BARDA) to invest in modern technologies that will lead to new vaccines and drugs against pandemic–capable viruses."
The Disease X Act of 2023 protects Americans by establishing a Disease X Medical Countermeasures Program at BARDA in the Department of Health and Human Services by the following:
- Providing funding of $40 million per year for five years specifically for Disease X MCMs;
- Clarifying the HHS may award contracts, grants, and cooperative agreements, or enter into other transactions, to promote the development of Disease X MCMs;
- Directing BARDA to accelerate and support the advanced research, development, and procurement of countermeasures and products to address Disease X threats; and
- Requiring products developed using the Disease X Program funding be substantially manufactured in the United States.
"This Act would be a major leap forward in our collective efforts to stay ahead of the ever-evolving threat landscape," commented Anita Cicero, Deputy Director of the Johns Hopkins Center for Health Security, in a press release.
"The Disease X Act of 2023 empowers BARDA to invest in modern technologies that will lead to new vaccines and drugs against pandemic–capable viruses."

Following the reduced demand for access to digital COVID-19 test and vaccine records through the epass.ny.gov portal and the official ending of the pandemic in the U.S., the NYS Wallet that hosts the Excelsior Pass Plus vaccine credential was disconnected as of July 28, 2023.
Since the NYS Wallet app will be removed from app stores, new users cannot log in and register for it.
The app was launched in March 2021 and was eventually used by 11.5 million people.
Media reported various consulting contracts, with over $200 million in spending, are currently the subject of an investigation by the state Office of the Inspector General.
Hazel Crampton-Hays, a spokeswoman for New York Gov. Kathy Hochul, said in a statement reported by Joshua Solomon with the Times Union, "Going forward, the state will use the knowledge gained from this project to improve how New Yorkers can use technology to access services and benefits."
The Excelsior Pass Plus and the NYS Wallet Apps provided a secure digital copy of vaccination records, including vaccine type, site, and date of vaccination, validated by the State of New York.
The State of New York says the data collected continues to be private and secure.
The state has set up an "Excelsior Help Desk," which can be reached at 844-699-7277 from 8 a.m. to 4 p.m., to assist those with questions or concerns about their vaccination records.
Internationally, the World Health Organization confirmed launching a digital health partnership on July 1, 2023, that will help protect citizens worldwide from ongoing and future health threats, including pandemics.

The Tico Times recently reported the Republic of Costa Rica increased its value-added tax (VAT) rate established in Law No. 9882.
Beginning on July 1, 2023, the local VAT was increased from 8% to 13% on the tourism industry.
The list of services for which VAT must be charged is available at the Ministry of Finance (www.hacienda.go.cr). A VAT is a consumption tax assessed on the value added in each good or service production stage.
The Association of Tourist Transporters of Costa Rica (ASTRONATUR) president describes it as “painful” for businesses and consumers, emphasizing that it will take a toll on the tourism sector’s finances.
Regarding health concerns, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions Travel Health Notice in April 2023 regarding a malaria outbreak in Costa Rica.
Other Costa Rica travel news regarding vaccinations, safety, and malaria and dengue outbreaks is posted by Vax-Before-Travel.