Search API
Invivyd, Inc. today announced additional positive initial data from its ongoing Phase 1 healthy volunteer clinical trial of its lead investigational monoclonal antibody (mAb) candidate, VYD222.
The results announced today add to the positive initial Phase 1 data reported by Invivyd in June 2023.
VYD222 is a broadly neutralizing, half-life extended mAb candidate in development for the prevention of symptomatic COVID-19 in vulnerable populations, such as immunocompromised people.
"We are pleased to see a favorable safety and tolerability profile as well as robust serum neutralizing titers against Omicron XBB.1.5 for all the VYD222 dose levels tested in our ongoing Phase 1 clinical trial," said Dave Hering, chief executive officer of Invivyd, in a press release on July 17, 2023.
"We believe these high neutralizing titer values and fold increases at this early timepoint are extremely encouraging as they indicate the potential for VYD222 to protect longer between doses and to provide additional protection from potential loss of neutralization activity as SARS-CoV-2 evolves."
The Phase 1 clinical trial of VYD222 enrolled 30 healthy volunteers across three dosing cohorts. In each cohort, participants were randomized 8:2 to VYD222 or placebo.
The initial Phase 1 data showed that a single administration of VYD222 was generally well-tolerated at all three dose levels tested, with no serious adverse events (SAEs) reported to date. At the middle VYD222 dose tested (2500 mg), geometric mean serum neutralizing titers were 9647.0 (95% CI: 6115.4, 15218.0) against Omicron XBB.1.5 at Day 7, with a geometric mean 92.82-fold rise (95% CI: 21.2, 406.6) from baseline to Day 7.
At the highest VYD222 dose tested (4500 mg), geometric mean serum neutralizing titers were 16864.7 (95% CI: 12825.5, 22176.1) against Omicron XBB.1.5 at Day 7, with a geometric mean 120.97-fold rise (95% CI: 31.4, 466.2) from baseline to Day 7.
The higher VYD222 dose levels tested in the Phase 1 clinical trial are designed to provide additional protection from potential loss of neutralization activity as SARS-CoV-2 evolves.
Analysis of the serum-neutralizing activity from samples collected at different time points across all dose cohorts in Phase 1 clinical trial is ongoing, as is detailed pharmacokinetic analysis and modeling.
Invivyd intends to use these analyses, combined with published clinical outcome data from prior clinical trials of vaccines and mAbs for the prevention of symptomatic COVID-19, including data from its Phase 2/3 clinical trial of adintrevimab for the prevention of COVID-19 (EVADE), to inform its VYD222 dosing strategy further.
Mr. Hering continued, "As a point of reference, we find it encouraging to observe that even our lowest VYD222 dose tested in our Phase 1 clinical trial resulted in higher titers against Omicron XBB.1.5 than the maximum titers against XBB.1.5 from investigational XBB-containing mRNA vaccines tested in humans that were shared at the FDA's recent vaccines advisory committee meeting."
"Higher VYD222 doses tested in our Phase 1 clinical trial have resulted, as expected, in higher titer levels that were well above those reported mRNA COVID-19 vaccine titer levels."
"We believe these initial Phase 1 clinical trial results support the potential for VYD222 to provide safe, meaningful, durable protection for vulnerable populations, such as immunocompromised people who may not generate adequate protection from COVID-19 vaccines, and we look forward to advancing VYD222 as fast as possible in collaboration with global regulators, starting with the FDA."
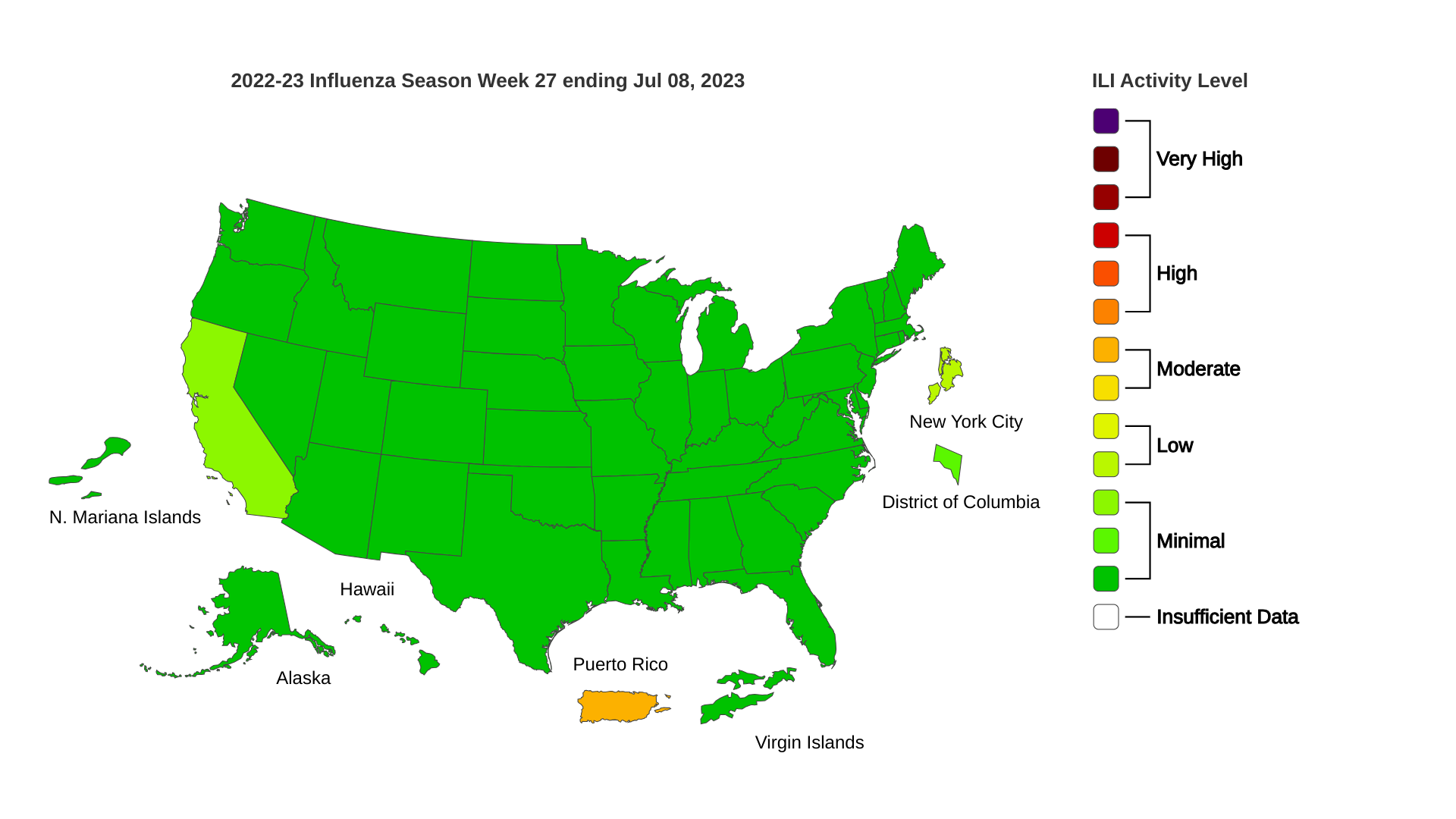
As of July 2023, mAbs targeting COVID-19 are in use globally.

While five World Health Organization (WHO) regions recently reported decreases in both cases and deaths, the African Region has reported an increase in fatalities, albeit from a relatively low baseline.
As of July 13, 2023, the WHO's Weekly epidemiological update Edition #151 revealed that during these 28 days, 57% (133 of 234) of countries and territories reported at least one COVID-19 case, which has been declining since mid-2022.
At the country level, the highest numbers of new 28-day cases were reported from the Republic of Korea (372 557 new cases; -22%), Australia (62 748 new cases; -59%), Brazil (56 744 new cases; -50%), New Zealand (38 949 new cases; +12%), and Singapore (28 333 new cases; -59%).
Furthermore, the WHO has recently Listed its twelfth COVID-19 vaccine.
In the U.S., COVID-19 trends by geographic area are posted by the CDC Data Tracker.
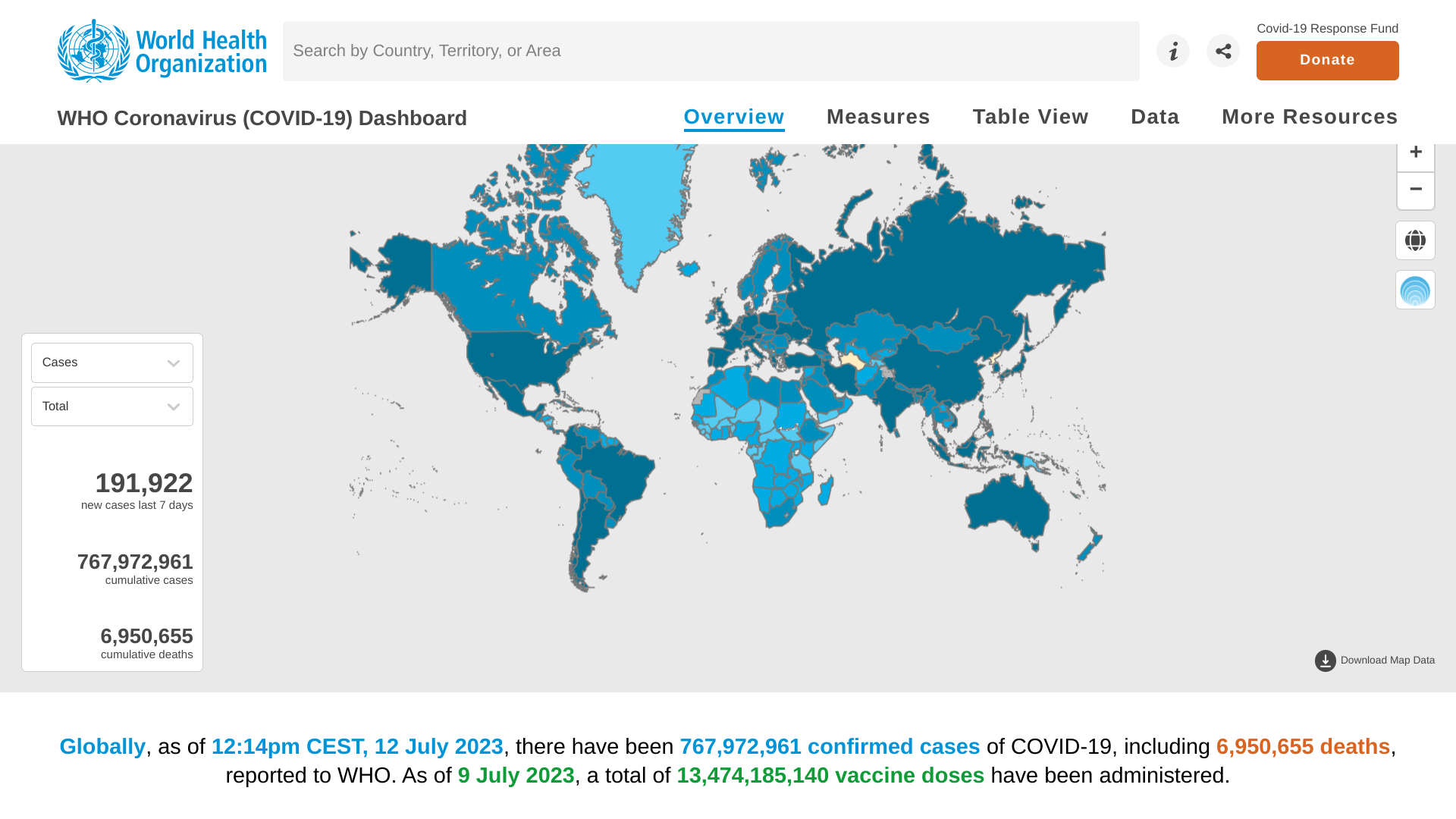
Since the last World Health Organization (WHO) situation report published in late June 2023, about seventeen countries have reported 316 new mpox cases and two new deaths have been reported by the WHO.
As of July 14, 2023, the WHO says there is a significant increase in mpox cases in the South-East Asia Region, driven by sustained community transmission in Thailand.
Available information on these Thailand cases shows that all patients are male, most from Bangkok without a travel history abroad in the 21 days before symptom onset. Around half of the new cases are among people living with HIV.
The U.S. CDC says the association of mpox cases with HIV infection highlights the need for a syndemic approach to care for HIV, sexually transmitted infections, and mpox in the context of comprehensive sexual health care.
However, mpox virus transmission continues at a low level in most countries reporting cases, and the main epidemiological and clinical characteristics of patients have remained stable over time, says the WHO.
From January 2022 through July 11, 2023, a cumulative total of 88,288 laboratory-confirmed cases of mpox, including 149 deaths, have been reported to WHO from 112 countries/territories/areas in all six WHO Regions.
And in the U.S., 1,243,378 mpox vaccine doses have been administered in 57 U.S. Jurisdictions.
As of July 15, 2023, Sexually Transmitted Disease vaccine news is posted by Precision Vaccinations.
The U.K. Health Security Agency (UKHSA) recently published HPR volume 17 issue 7, which shows measles cases to increase in 2023.
Between January and June 30, 2023, there have been 128 measles cases, compared to 54 cases in 2022.
With 66% of the cases detected in London.
The risk in London is primarily due to low vaccination rates, particularly in some areas where coverage of the first measles vaccine dose at two years of age is as low as 69.5%.
However, the UKHSA's measles epidemic risk assessment across the U.K. is considered low.
The assessment also concludes that there is a high risk of cases linked to overseas travel leading to outbreaks in specific population groups such as young people and under-vaccinated communities.
Dr. Vanessa Saliba, UKHSA Consultant Epidemiologist, said in a press release on July 14, 2023, "Measles spreads very easily but is preventable."
"Nobody wants to see their child or loved ones sick with measles, or put others more vulnerable, like babies, at risk."
Measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
To address these issues, NHS England has launched a targeted national campaign to encourage measles vaccine uptake, including targeted outreach work in London for those identified as at high risk and communities with the lowest vaccination uptake.
As of July 7, 2023, a total of 18 measles cases were reported by 12 jurisdictions in the United States.
The U.S. CDC's Travel Health Notice, issued in late June 2023, says all international travelers, including infants 6–11 months of age and preschool-aged children, should be fully vaccinated against measles.
The CDC's Global Level 1 - Practice Usual Precautions notice did not include the United Kindom.

The Houston Health Department recently reported a syphilis outbreak has significantly impacted women in eastern Texas and continues to expand in 2023.
As of July 13, 2023, there has been a 128% increase in syphilis cases among women and a nine-fold rise in congenital syphilis in Harris County.
In response to the syphilis outbreak, Houston's Health department is waving all clinical fees for sexually transmitted infections at its health centers. Untreated syphilis during pregnancy can result in a stillbirth or a baby's death soon after birth.
Syphilis testing is recommended in Texas at a woman's first prenatal visit, during the third trimester, and at delivery.
"It is crucial for pregnant women to seek prenatal care and syphilis testing to protect themselves from an infection that could result in the deaths of their babies," said Marlene McNeese Ward, deputy assistant director in the department's Bureau of HIV/STI and Viral Hepatitis Prevention, in a related press release.
"A pregnant woman needs to get tested for syphilis three times during pregnancy."
Statistics from the department indicate new syphilis infections rose from 1,845 in 2019 to 2,905 in 2022, a 57% increase.
Cases among women totaled 674 cases in 2022, up from 295 cases in 2019.
And congenital syphilis soared from 16 cases in 2016 to 151 cases in 2021.
Since reaching a historic low in 2001, the rate of syphilis has increased almost every year in the U.S., increasing 28.6% from 2020 to 2021.
Nationwide, men account for the most cases of syphilis.
In most cases, syphilis goes undetected because the signs and symptoms are misinterpreted or unnoticed.
If untreated, Treponema Pallidum, the bacterium that causes syphilis, remains in the body and begins to damage the internal organs, including the brain, nerves, eyes, heart, blood vessels, liver, bones, and joints, says the U.S. CDC.
As of July 15, 2023, the U.S. FDA has not approved a syphilis vaccine.
A systematic review and meta-analysis published today in Antimicrobial Resistance and Infection Control journal show that influenza vaccination is associated with significantly reduced antibiotic use.
The study focused on data from randomized controlled trials (RCT) and observational studies.
The RCTs showed that the effect of influenza vaccination on the number of antibiotic prescriptions or days of antibiotic use (Ratio of Means (RoM) 0.71, 95% CI 0.62–0.83) is stronger compared to the effect of pneumococcal vaccination (RoM 0.92, 95% CI 0.85–1.00).
These studies also confirm a reduction in the proportion of people receiving antibiotics after influenza vaccination (Risk Ratio (RR) 0.63, 95% CI 0.51–0.79).
And the effect of influenza vaccination in the European and American regions ranged from RoM 0.63 and 0.87 to RR 0.70 and 0.66, respectively.
However, the evidence from observational studies supports these findings but presents a less consistent picture.
Announced on July 14, 2023, this data supported the use of influenza vaccination as an important public health intervention to reduce antibiotic use and possibly control antimicrobial resistance.
In the northern hemisphere, the 2023-2024 flu season is forecasted to begin in the fall, with an ample supply of influenza vaccines available at most clinics and pharmacies in the U.S.