Search API
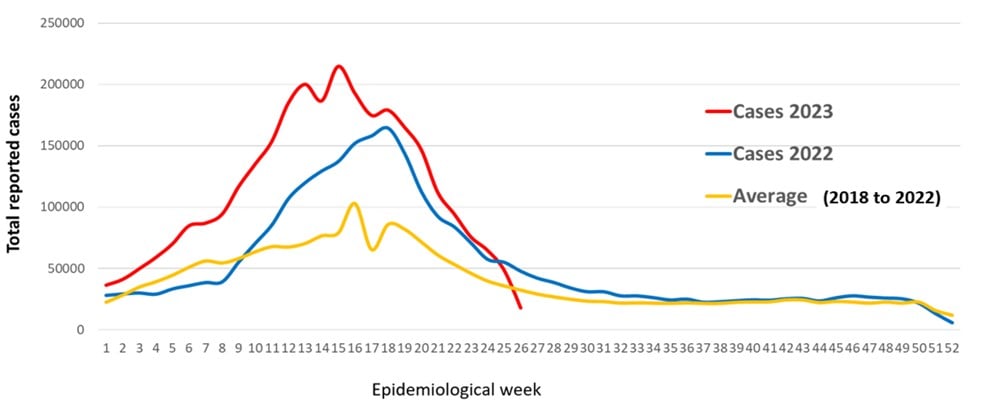
Since the beginning of 2023, dengue outbreaks of significant magnitude have been recorded in the WHO Region of the Americas, with close to three million suspected and confirmed cases of dengue reported as of July 19, 2023.
This total surpassed the 2.8 million cases of dengue registered for the entire year of 2022.
The WHO has assessed the risk of dengue as high at the regional level since all four dengue virus serotypes are present in the Region of the Americas.
One example of these dengue outbreaks is Costa Rica.
As of EW #25, there have been 2,712 reported dengue cases, which is 16% higher compared to the same period in 2022 and 19% higher compared to the average of the last five years.
Almost half of the world’s population, about 4 billion people, live in areas with a risk of dengue. Dengue is often a leading cause of illness in areas with risk, says the U.S. CDC.
In the U.S., Florida continues to report both locally-acquired and travel-related dengue cases in July 2023.
Symptoms can be mild or severe for people who get sick with dengue. However, severe dengue can be life-threatening within a few hours and often requires care at a hospital.
Dengue viruses are spread to people through the bite of an infected Aedes mosquito.
The WHO does not recommend travel restrictions for countries in the Americas experiencing dengue epidemics based on the currently available information.
From a disease prevention perspective, two approved dengue vaccines (Qdenga and Dengvaxia) are available in certain countries in 2023.
VBI Vaccines Inc. today announced that the PreHevbri® vaccine is now available in the Netherlands and Belgium for active immunization against infection caused by all known subtypes of the hepatitis B virus (HBV) in adults.
It is the only approved 3-antigen hepatitis B vaccine for adults in the Netherlands and Belgium.
As part of the partnership announced in September 2022, PreHevbri is available through France-based Valneva SE's commercial infrastructure and distribution networks.
PreHevbri (PreHevbrio™, Sci-B-Vac®) can be expected that hepatitis D will also be prevented by immunization with PreHevbri as hepatitis D does not occur without hepatitis B infection.
Jeff Baxter, President and CEO of VBI, commented in a press release on July 19, 2023, "Over the next several months, we expect to continue to expand global access to PreHevbri with additional market launches as we work to make an impact in the fight against hepatitis B worldwide."
This HBV vaccine is currently available in the U.S., Europe, the U.K., and Israel.
Hepatitis B is one of the world's most significant infectious disease threats, with more than 290 million people infected globally. It is the leading cause of liver disease, about 25%, and is tough to cure, says the U.S. CDC.
Furthermore, women with hepatitis B can transmit the virus to their newborns during birth. If not vaccinated, 90% of infants infected with HBV at birth will progress to chronic HBV infections.

Emergex Vaccines Holding Limited today announced the successful completion of naNO-COVID, a Phase I clinical trial investigating the safety and reactogenicity of CoronaTcP™ in healthy volunteers.
CoronaTcP is Emergex's multi-target T cell-priming set-point product, designed to be broadly effective against diseases caused by betacoronaviruses, including SARS-CoV-1 and SARS-CoV-2 variants.
Cellular analyses demonstrated that CoronaTcP (two doses administered at Day 0 and 21) was able to activate virus-specific CD8+ T cells, with a significant increase in frequencies of CoronaTcP-specific CD8+ CD137+ CD69+ cells following in vitro antigenic stimulation in both low and high dose CoronaTcP groups at Day 35 post-treatment.
Significant changes were also observed for several virus-specific CD8+ memory subsets.
Professor Thomas Rademacher, Co-Founder and Chief Executive Officer of Emergex, said in a press release on July 19, 2023, Demonstrating that our platform has an acceptable safety profile and successfully mobilizes specific T cells that may elicit broad and long-term immune memory, validates our approach."
"By improving T cell-based immunity, we can enhance any previous immune status. We are delighted that this first assessment of treatment against infectious diseases for clinical use, based solely on a T cell response, was successful."
Overall, Phase I trial data validate Emergex's T cell-based approach to protection against RNA viruses and confirm the platform's potential using this innovative technology, supporting the investigation of other T cell-priming immune set-point candidates from the same platform.
In secondary immunogenicity analyses, several participants seroconverted during the trial (due to exposure to SARS-CoV-2) but had mild symptoms, confirming that CoronaTcP does not worsen an acute episode of COVID-19.
The naNO-COVID trial was a Phase I double-blind, randomized, base particle-controlled, single-center study designed to evaluate the safety and reactogenicity of two intradermal injections of an anti-Betacoronavirus candidate.

The U.S. Centers for Disease Control and Prevention (CDC) today announced an Emergency Preparedness and Response COCA Call on July 20, 2023, where participants will learn how to prevent, diagnose, and treat malaria.
And how the biology of the pathogen contributes to the clinical management of the disease,
And how CDC and state and local health departments respond to the locally acquired mosquito-borne malaria cases in Florida and Texas.
Previously, the CDC issued a Health Alert Network Health Advisory on June 26, 2023, to share information on the recent identification of locally acquired, mosquito-transmitted malaria cases (P. vivax) in Florida and Texas.
Although these are the first documented instances of locally transmitted malaria in the U.S. since 2003, approximately 2,000 malaria cases are diagnosed and treated in the U.S. each year.
Malaria cases are primarily confirmed in individuals returning from travel to malaria-endemic countries such as Brazil and Cuba.
These malaria infections present a potential risk of subsequent transmission domestically since Anopheles mosquitoes, capable of transmitting malaria, are broadly distributed across the United States.
For example, locally-infected and travel-related malaria cases in Florida were confirmed in July 2023.
During this COCA call on July 20, 2023, at 2:00 PM – 3:00 PM ET, at Webinar Link: https://www.zoomgov.com/j/1618112526, will learn CDC and state and local health departments are responding to the locally acquired mosquito-borne malaria cases in the U.S.
Unfortunately, the U.S. government has not approved a malaria-prevention vaccine as of July 18, 2023.

Florida Health's latest Mosquito-Borne Disease Surveillance report indicates the recent malaria outbreak in the Sarasota area has continued into the summer of 2023.
As of July 15, 2023, Florida Health'w week #28 reported confirmed the seventh locally-acquired malaria case in the Sarasota area since May 2023. The Plasmodium species reported were Plasmodium vivax.
And state-wide, there have been 26 travel-related malaria cases reported in Broward (5), Duval, Hillsborough (4), Lee, Leon (2), Miami-Dade (5), Orange (2), Osceola, Pinellas (3), Sarasota, and Volusia counties this year.
The majority of travel-associated malaria cases in Florida were in people who had recently visited Africa.
Malaria still threatens international travelers, military personnel, and U.S. citizens living and working abroad, says the Centers for Disease Control and Prevention (CDC).
According to the World Health Organization World Malaria Report, the global number of malaria outbreaks reached about 240 million cases, with over 600,000 related fatalities in 2021.
As of July 18, 2023, the U.S. CDC says the bite of an infective female Anopheles mosquito spreads malaria.
The disease can cause fever, chills, and flu-like illness. If it is not treated, it can cause severe complications and death.
The CDC has issued malaria alerts for malaria-endemic countries, including Costa Rica, but not Florida.
Various antimalarial treatments are approved by the CDC in 2023, but not no malaria vaccines.
As of July 5, 2023, twelve African countries are set to receive 18 million doses of GSK's Mosquirix recombinant vaccine over the next two years.

Pfizer Inc. and Flagship Pioneering, Inc. today announced a partnership to create a new pipeline of innovative medicines. The focus will be addressing unmet needs within Pfizer's core strategic areas of interest, including in broad patient populations and diseases with high potential to benefit from diverse technology platforms and modalities.
Pfizer will fund and have options to acquire development programs.
Under the terms of the novel agreement, Flagship and Pfizer will each invest $50M upfront to explore opportunities to develop ten single-asset programs by leveraging Flagship's ecosystem of more than 40 human health companies and multiple biotechnology platforms.
To date, Flagship has deployed over $3.4 billion in capital toward the founding and growth of its pioneering companies alongside more than $26 billion of follow-on investments from other institutions.
Per the new agreement, Flagship and its bioplatform companies can receive up to $700M in milestones and royalties for each successfully commercialized program.
"At Pfizer, we are expanding our efforts to pursue potential breakthrough science with unique approaches and funding mechanisms designed to leverage the dynamic scientific ecosystem," said Mikael Dolsten, M.D., Ph.D., Chief Scientific Officer and President, Worldwide Research, Development and Medical of Pfizer, in a press release on July 18, 2023.
"This collaboration is an exciting opportunity for Pfizer to bring deep scientific expertise and apply our development and regulatory strength to Flagship's diverse portfolio of technology platforms, translating early-stage innovation to potential medicines."

Vaxxinity, Inc. today announced new data from an early-stage clinical trial demonstrating that antibodies derived from its investigational immunotherapeutic for Parkinson's disease, UB-312, slows the seeding of alpha-synuclein (aSyn) in cerebrospinal fluid (CSF) of patients as demonstrated using multiple target engagement assays.
These data signify that UB-312 has established clear target engagement in Parkinson's disease (PD) patient CSF.
Jean-Cosme Dodart, Ph.D., SVP of Research at Vaxxinity, commented in an email, “The more data we see from our UB-312 program, the more excited I get for the future of this vaccine and its potential positive impacts for patients with PD or other a-synucleopathies."
"Demonstrating target engagement in PD patients immunized with UB-312 is an exciting milestone which encourages us to push this program further into clinical development.”
UB-312 is a UBITh®-enhanced synthetic peptide-based vaccine designed to target aggregated forms of aSyn, the toxic species that underlies PD and other synucleinopathies.
"Our candidate has shown target engagement of the toxic species of alpha-synuclein in patients, demonstrating not only proof of our technology platform but also proof of the mechanism of our vaccine-derived antibodies specifically engaging with the toxic target in vivo," said Mei Mei Hu, CEO of Vaxxinity, in a press release on July 17, 2023.
"Showing target engagement has always been a key challenge to overcome in neurodegeneration and is of critical importance when demonstrated – a milestone worth celebrating."
"It is beyond our expectation to see this in our Phase 1 clinical trial."
"We are endlessly grateful to the patients who participated and to The Michael J. Fox Foundation and our collaborators for their work on these cutting-edge assays that supported this breakthrough."
Last month, Vaxxinity announced clinical data from Part B of its Phase 1 clinical trial of UB-312 demonstrating that UB-312 was well-tolerated and induced anti-aSyn antibody responses in participants with early PD and that antibodies were detectable in the CSF.
As part of this trial, The Michael J. Fox Foundation (MJFF) funded a 2-year collaborative project between Vaxxinity, the Mayo Clinic, and UTHealth Houston to analyze CSF collected from patients and to conduct exploratory research to characterize the anti-aSyn antibodies produced after UB-312 administration and assess target engagement.
Mark Frasier, Ph.D., Chief Scientific Officer of MJFF, commented, "Integration of critical biomarker insight into therapeutic development programs is essential for building confidence in the treatment approach and designing informative trials. We're pleased to support efforts of this kind that can have a major impact for people with Parkinson's disease."

AstraZeneca and Sanofi's today announced Beyfortus™ (nirsevimab-alip) has been approved in the United States for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD) in newborns and infants born during or entering their first RSV season.
And for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Beyfortus is the first preventive option approved to protect a broad infant population, including those born healthy at term or preterm or with specific health conditions that make them vulnerable to severe RSV disease.
Furthermore, the single dose can be flexibly administered at the beginning of the RSV season or at birth for those born during the RSV season.
The companies confirmed on July 17, 2023, Beyfortus will be available for the 2023-2024 RSV season in the U.S.
Iskra Reic, Executive Vice President, Vaccines and Immune Therapies, AstraZeneca, said in a press release, "Beyfortus represents an opportunity for a paradigm shift in preventing serious respiratory disease due to RSV across a broad infant population in the U.S."
"The science that Beyfortus is built on demonstrates AstraZeneca's continued leadership in addressing the needs of the most vulnerable populations and reducing the burden on healthcare systems."
The Food and Drug Administration (FDA) approval followed the unanimous vote by the Antimicrobial Drugs Advisory Committee on the favorable benefit-risk profile of Beyfortus. It was based on the extensive clinical development program for Beyfortus, spanning three pivotal late-stage clinical trials.
Beyfortus was generally well tolerated, with a favorable safety profile consistent across all clinical trials.
Beyfortus has already been approved in the European Union and the United Kingdom.
According to the U.S. Centers for Disease Control and Prevention, RSV is a very contagious virus that can lead to serious respiratory illness in infants. Two out of three infants are infected with RSV during their first year of life, and almost all infants are infected by their second birthday.
The JAMA Network's Original Investigation in 2022 reported 6,549 respiratory fatalities were associated with RSV each year, including 96 (95% CI, 92-99) among children younger than one year.
RSV mAbs (Synagis) have been approved in the U.S. since 1998. Additionally, RSV vaccines have recently been approved by the FDA.


