Search API
GlaxoSmithKline Plc filed a lawsuit alleging Pfizer Inc.'s respiratory syncytial virus (RSV) vaccine infringes on four patents for AREXVY™ RSV OA, a single dose, monovalent RSV vaccine.
AREXVY was the first RSV vaccine approved by the U.S. Food and Drug Administration.
Pfizer's RSV vaccine was the second one approved.
Bloomberg Law reported on August 2, 2023, the GlaxoSmithKline Biologicals SA et al v. Pfizer Inc. complaint was filed in the U.S., District of Delaware.
"Upon information and belief, Pfizer knowingly uses GSK's claimed inventions in ABRYSVO™, a bivalent prefusion F subunit vaccine, without permission," wrote GSK.
GSK is seeking a jury trial, monetary damages, and is asking a judge to prevent Pfizer from selling Abrysvo to adults 60 and older in the U.S.
The U.S. Centers for Disease Control and Prevention announced on June 29, 2023, and July 21, 2023, the use of RSV vaccines for people ages 60 years and older, requires shared clinical decision-making.

The U.S. Department of State officially launched the Bureau of Global Heath Security and Diplomacy.
The Bureau’s overarching mission is to fortify the global health security architecture to effectively prevent, detect, control, and respond to infectious diseases, including HIV/AIDS, wrote the Secretary of State on August 1, 2023.
By leveraging and coordinating U.S. foreign assistance, the Bureau aims to foster robust international cooperation, enhancing protection for the United States and the global community against health threats through strengthened systems and policies.
To ensure U.S. leadership is sustained moving forward, the Bureau will provide a unified voice of leadership on global health security and diplomacy, combining strengths, functions, personnel, and resources from various offices.
Ambassador-at-Large Dr. John N. Nkengasong will lead the Bureau.
This new Bureau will seamlessly integrate global health security as a core component of U.S. national security and foreign policy, underscoring the Department of State’s commitment to advancing human health worldwide, wrote the State Department.
The State Department also issued security notices for most countries, found at this link.

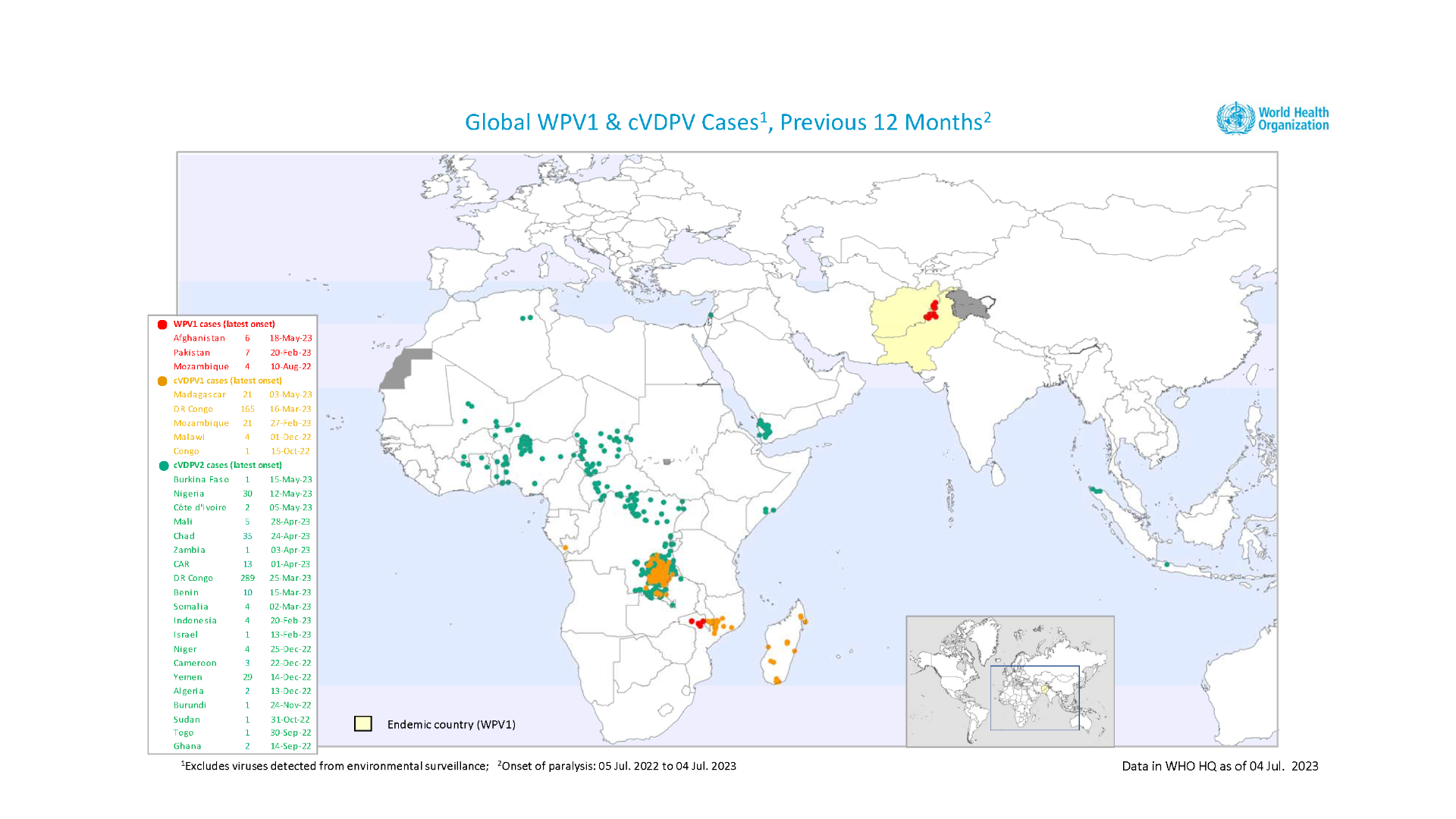
With 117 confirmed cases of circulating variant polioviruses in the WHO African Region this year, the Africa Regional Certification Commission recently urged countries to urgently address gaps in polio immunity to avert future outbreaks.
In recent years, polioviruses have paralyzed hundreds of African children, says the WHO.
On July 28, 2023, the WHO announced the detection of a circulating vaccine-derived poliovirus type 2 (cVDPV2) in two acute flaccid paralysis (AFP) cases and two asymptomatic healthy children community contacts from the Hagadera camp in Kenya.
The U.S. NIH says AFP surveillance is the standard for detecting cases of poliomyelitis in anyone under 15 years of age.
On July 25, 2023, Madagascar launched polio vaccinations for nearly 18 million children, adolescents, and adults in the priority regions of Analamanga, Vakinankaratra, and Alaotra Mangoro. Madagascar has reported 79 cases of cVDPV1 in 2023. Forty-five of them were cases of AFP, including 198 environmental samples.
On July 4, 2023, the Ministry of Health of the United Republic of Tanzania notified the WHO of the detection of cVDPV2. The virus was isolated from an AFP case in the Rukwa region, southwestern Tanzania.
To alert international travelers, the U.S. Centers for Disease Prevention and Control (CDC) included various African countries in its Global Polio Travel Health Advisory on July 10, 2023.
Furthermore, the U.S. was added to about thirty countries where polio was recently identified. In the U.S., poliovirus was confirmed in 2022 and 2023 in wastewater samples.
The CDC recommends polio vaccinations before visiting outbreak areas.
Merck & Co. today announced its human papillomavirus (HPV) vaccines sales increased 47% to reach $2.5 billion, and if you exclude the Impact of Foreign Exchange, sales actually grew by 53%.
Merck's GARDASIL 9® is a vaccine indicated in men and women, 9 through 45 years of age, to prevent cancers caused by the HPV. It has been available in the United States since late 2016.
The original GARDASIL® vaccine consists of 4 proteins of HPV types 6, 11, 16, and 18. is available in other countries.
According to the World Health Organization, HPV vaccination programs that began pre-pandemic reached the same number of women in 2022 as in 2019, with mean coverages reaching 67% in high-income countries and 55% in low- and middle-income countries.
Millions of men and women aged 27–45 may still benefit from HPV vaccination, says Merck.
"We continue to make great progress as we advance our broad and deep pipeline, raise the bar of innovation, and bring forward leading-edge science to save and improve lives around the world," said Robert M. Davis, chairman, and chief executive officer of Merck, in a press release issued on August 1, 2023.
HPV is a double-stranded DNA virus that belongs to the Papillomaviridae family. There are over 100 subtypes of HPV, characterized as high-risk or low-risk. And it is the most common sexually transmitted infection worldwide.
From a clinical perspective, HPV vaccinations reduce anal HPV infection and anal intraepithelial neoplasia (AIN).
A study published on May 31, 2023, concluded there is strong evidence for high vaccine efficacy against anal HPV infection and AIN in HIV-negative individuals vaccinated at age ≤26 years.

Consumers have recently expressed a desire to take a more proactive approach to Alzheimer's disease (AD) screening and a willingness to explore earlier diagnoses.
To meet that new trend, Quest Diagnostics today announced the availability of the AD-Detect™ Test for AD, the first blood test available for consumer purchase that helps assess the potential risk of developing AD based on a brain protein that contributes to the condition.
Quest's AD-Detect is a screening test that uses plasma, the liquid component of blood, from a single blood draw to evaluate levels of amyloid beta proteins to help detect early signs associated with the risk of developing AD.
Amyloid beta proteins are known to accumulate and form plaques in the brain, which are linked to the progression of Alzheimer's disease. AD-Detect evaluates the ratio of two peptides of amyloid beta, Aβ42, and Aβ40, in plasma.
"We are seeing much attention on emerging therapies for Alzheimer's disease, but with new treatment options will come the need to make screening and diagnosis more widely available. Blood tests like AD-Detect hold incredible potential to make Alzheimer's disease risk assessment both accessible and convenient," said Michael K. Racke, M.D., Medical Director of Neurology, Quest Diagnostics, in a press release on July 31, 2023.
The new consumer-initiated test utilizes the same expertise and technology as Quest's clinical AD-Detect Amyloid Beta 42/40 Ratio test, an analytically validated blood test that aids in assessing the risk of AD, which the company launched for physician ordering in early 2022.
As of August 1, 2023, there are no Alzheimer's disease vaccines available in the U.S.

The U.S. Fish and Wildlife Service (FWS) Incident Command Team recently confirmed implementing conservation strategies to help California condors in light of the Highly Pathogenic Avian Influenza (HPAI) bird flu outbreak.
As of July 28, 2023, over twenty-one Condors have died related to HPAI infections this year.
In May 2023, the United States Department of Agriculture's Agricultural Research Service announced the emergency use of a HPAI vaccine candidate to prevent additional deaths of California Condors.
The California Condor Vaccination Trial continued will continue into September 2023.
Blood samples from 13 birds will be collected at 21 and 42-days following vaccination to evaluate the immune response from two different vaccination approaches.
The first sample will be collected on August 8.
From a recovery perspective, three condors were transferred to the release site in Arizona to reacclimate to their home. A release date will be determined based on their behavior and weather.
The fourth bird that survived also has immunity to HPAI and will be released later as he is currently re-growing molted flight feathers.
The California Condor Recovery Program continues to implement standard operations, and we are hopeful this will include the release of juveniles in 2023. However, due to the dynamic nature of HPAI outbreaks and logistics around potential future vaccinations, adjustments will be made accordingly, wrote the FWS.
The ongoing bird flu outbreak reached Europe, Asia, and Russia in 2023.
Furthermore, the U.S. government has already approved one bird flu vaccine (Audenz™) for people and invested in vaccine candidates should a pandemic occur.

The U.S. CDC Advisory Committee on Immunization Practices (ACIP) is meeting on August 3, 2023, regarding the proposed recommendation for Beyfortus™ (Nirsevimab-alip), the first approved extended half-life monoclonal antibody (mAB) offering passive immunization to prevent lower respiratory tract infections (LRTI) caused by the respiratory syncytial virus (RSV).
This ACIP meeting draft agenda, from 11:00 am – 3:30 pm EDT, includes presentations on Feasibility/implementation plans for monitoring the safety and effectiveness of this RSV prevention drug and second-season clinical considerations.
The webcast link for this open-to-the-public digital meeting is here.
John Farley, M.D., M.P.H., director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, commented on July 17, 2023, “Today’s approval (Beyfortus) addresses the great need for products to help reduce the impact of RSV disease on children, families, and the health care system.”
The FDA previously approved the Synagis® (Palivizumab) RSV mAb in 1998.
RSV is a virus that causes acute respiratory infection in individuals of all age groups. While most infants and young children experience mild, cold-like symptoms, some infants, especially with their first infection. RSV is transmitted from person to person through close contact with someone who is infected.
In most parts of the U.S., RSV circulation is seasonal, typically starting in Florida during the fall and peaking in the winter.



