Search API
The Access to Advanced Health Institute (AAHI) today announced that it has received an $18 million award from the National Institutes of Health (NIH) to develop a temperature stable, single-dose, RNA chikungunya vaccine candidate.
The NIH award disclosed on August 3, 2023, supports the development, preclinical testing, and human clinical evaluation of a vaccine that meets an increasingly urgent need for a reliable, abundant supply.
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus (CHIKV), says the U.S. CDC.
Chikungunya outbreaks are significant causes of morbidity and mortality in Asia, Africa, and Latin America, for which no vaccine is currently approved.
From 2006–2021, 4,590 chikungunya cases in travelers were reported in the U.S.
Several vaccine candidates are conducting late-stage clinical trials, such as Valneva SE's VLA1553, a monovalent, single-dose, live-attenuated vaccine candidate.
AAHI's approach to an RNA vaccine against chikungunya differs from the RNA vaccines the U.S. FDA currently approves to prevent other diseases.
“This project will demonstrate the use of RNA vaccine technology to avoid some of the classic manufacturing challenges in the large-scale manufacture of live-attenuated vaccines,” said Emily Voigt, Ph.D., Principal Scientist, AAHI RNA Platform Lead, and Co-Principal Investigator for the award, in a press release.
Unlike other RNA vaccines, this candidate will generate a live "attenuated" virus that could induce strong and long-lasting immune protection against this mosquito-borne disease.
The new 5-year project builds upon work supported by the NIH (R43AI127053) for AAHI's proof-of-concept ground-laying work, which demonstrated that a liquid presentation of this live-attenuated chikungunya RNA vaccine candidate elicited strong immune responses in animals after a single dose, protecting them from mortality and joint swelling after being challenged with the virus (Voigt et al. 2021).
AAHI is a nonprofit biotech research institute located in Seattle, Washington, that combines the high-quality science of an academic research organization with the product development capabilities of a biotech company to help combat some of the world's deadliest diseases, including infectious diseases

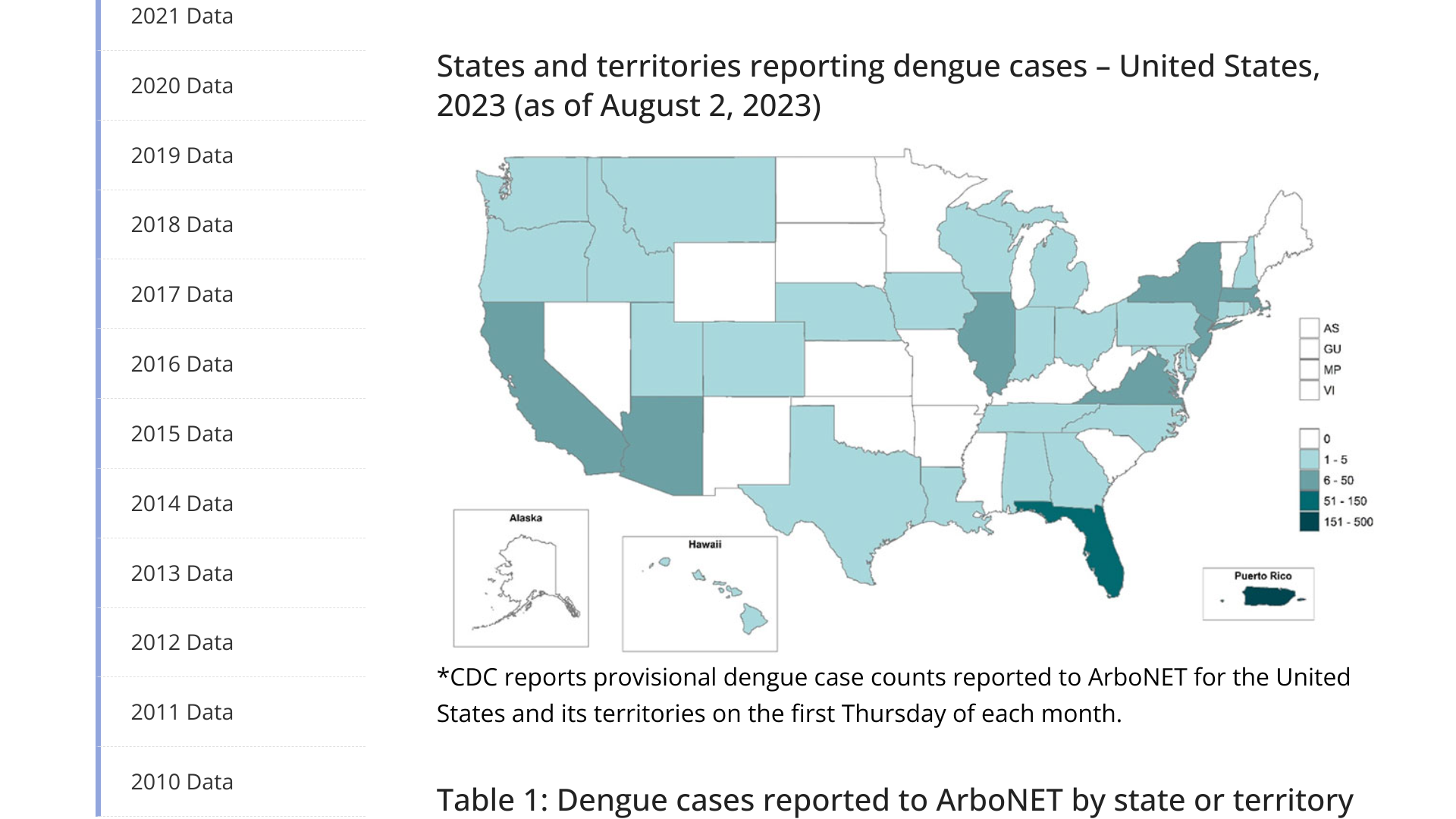
The U.S. Centers for Disease Control and Prevention (CDC) today reported the global dengue outbreak is impacting the United States.
On August 2, 2023, the CDC provisionally confirmed the U.S. States reported 225 dengue cases, and Territories reported 315.
For all of 2022, the CDC reported, the CDC reported 2,016 dengue cases.
From a state perspective, the Florida Health Department reported as of week #30 in 2023, there had been 147 travel-associated dengue cases. The majority (98) of travel cases were related to Cuba.
Florida has also reported six locally acquired dengue cases in 2023.
To alert travelers to their dengue health risk, the CDC recently issued Travel Health Notices for the Americas (2023), Africa/Middle East (July 21, 2023), Costa Rica, and Asia/Pacific Islands (July 25, 2023).
The CDC says dengue is a vaccine-preventable disease. As of August 3, 2023, two dengue vaccines are in use worldwide.
The World Health Organization (WHO) today reported (Edition 154) that during this recent 28-day period, 46% (107 of 234) of countries and territories reported at least one COVID-19 case, a proportion that has been declining since mid-2022.
While five WHO regions have reported decreases in both cases and deaths, the Western Pacific Region has reported an increase in patients and a decline in fatalities.
Globally, over 3,100 COVID-19-related deaths were reported between July 3 and 30, 2023.
Click on these action circles to learn more about the COVID-19 pandemic.
As of August 3, 2023, the WHO has LIsted 12 COVID-19 vaccines that are available in certain countries.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) is meeting today to review Respiratory Syncytial Virus (RSV) Maternal/Pediatric vaccine and a long-acting monoclonal antibody.
On August 3, 2023, Dr. Grace Lee is leading the ACIP meeting agenda, which includes, but is not limited to, the following presentations:
-
Introduction - Dr. S Long
-
EtR summary for nirsevimab - Dr. J Jones
-
Nirsevimab implementation considerations - Dr. G Peacock
-
Clinical considerations for nirsevimab & Workgroup considerations / proposed recommendations - Dr. J Jones
At around 2 pm ET today, the ACIP is scheduled to vote on two recommendations.
Previously, the U.S. Food and Drug Administration approved Beyfortus (nirsevimab-alip) for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
In the U.S., the RSV season generally starts in Florida in the fall. As of August 3, 2023, there have not been any RSV outbreaks reported this year.
The ACIP unanimously recommends routine use of Beyfortus™ to protect all infants below 8 months of age. The committee also voted unanimously to include Beyfortus in the Vaccines for Children program, supporting equitable access for all eligible infants.

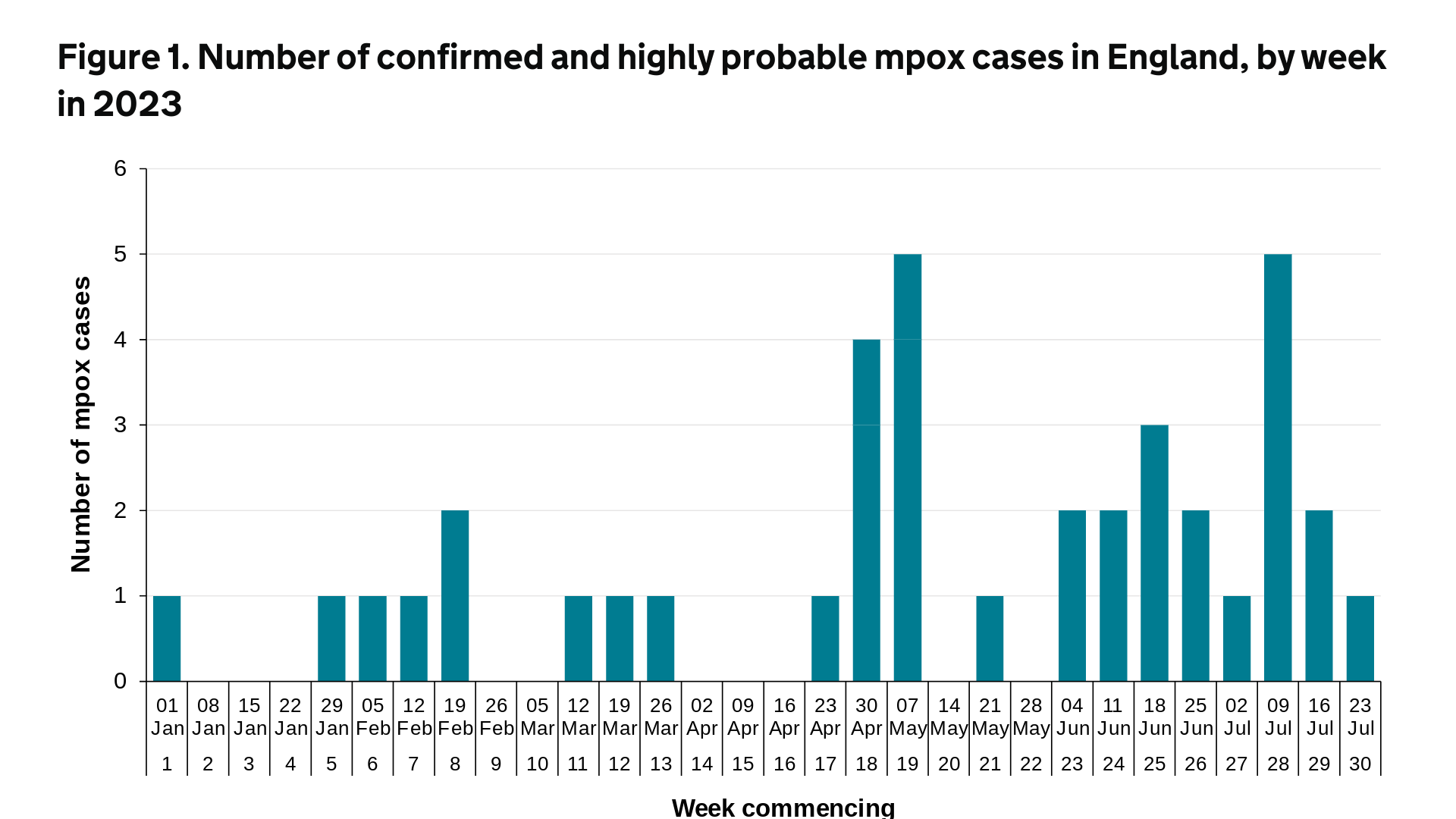
The UK Health Security Agency (UKHSA) today published Research and Analysis, Mpox outbreak: epidemiological overview, as of August 3, 2023.
Up to December 31, 2022, there were 3,732 confirmed and highly probable mpox cases reported in the UK. Of these, 3,553 were in England, 34 were in Northern Ireland, 97 were in Scotland, and 48 were in Wales.
In 2023 (up to July 31, 2023), a further 39 cases of mpox were reported in the UK.
Of these, 38 were in England. The most recent mpox cases, seen from April 2023 onwards, have been focused in London.
In reaction, mpox vaccinations have been extended in London because of a spike in infections, says the UKHSA.
The leading mpox vaccine is Bavarian Nordic's JYNNEOS® (MVA-BN) vaccine, which is based on a live, attenuated vaccinia virus.
Bavarian Nordic A/S today announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA) placed a new order valued at USD 120 million, primarily covering the manufacturing of new bulk product for the Company's JYNNEOS® smallpox/mpox vaccine.
The bulk product, representing USD 96 million of the contract value, will be manufactured and invoiced in 2023 and will only partly restore the inventory used to manufacture vaccines in response to the global mpox outbreak that began in May 2022.
Nearly 5.5 million JYNNEOS doses have been manufactured for the U.S. government throughout 2022 and 2023, and replenishment of the bulk inventory is necessary to fulfill the Company's long-term commitment to deliver a freeze-dried version of the vaccine for U.S. smallpox preparedness.
In addition, Bavarian Nordic will manufacture and supply additional liquid-frozen vaccine doses in 2023, valued at USD 3 million.
The agreement includes additional services totaling USD 21 million, of which the majority will be received in 2024 and 2025.
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on August 3, 2023, "The U.S. government's foresight enabled us last year to rapidly respond to the global mpox outbreak by converting the readily available bulk product into final vaccine dose."
"Together with our U.S. manufacturing partner, we have completed manufacturing all doses ordered by the U.S. government during the mpox outbreak."
"However, maintaining the readiness to respond to future health crises is essential, and this new contract will enable us to deliver on the contract for a freeze-dried version of the vaccine, awarded to us by the U.S. government back in 2017, which aims to strengthen the nation's preparedness against smallpox."
Since 2003, Bavarian Nordic has worked with the U.S. government on the development, manufacturing, and supply of a non-replicating smallpox vaccine. The JYNNEOS (MVA-BN, IMVANEX®) vaccine has been deployed globally since 2022.
In 2023, mpox outbreaks have been reported Africa, the Americas, Chicago, China, Denver, France, Japan, London, New York, Portugal, South Korea, and Spain. Furthermore, mpox breakthrough cases in vaccinated people have been confirmed in 2023.

Merck today announced that the U.S. Food and Drug Administration (FDA) approved an expanded indication for the ERVEBO® vaccine, which is now indicated for preventing disease caused by Zaire ebolavirus in individuals 12 months of age and older.
ERVEBO was previously approved for use in individuals 18 and older.
This Ebolavirus vaccine does not protect against other species of Ebolavirus (Sudan) or Marburgvirus.
As of March 2023, over 500,000 doses of ERVEBO had been delivered to a stockpile administered by the International Coordinating Group on Vaccine Provision.
"Ebola virus disease is contagious and potentially deadly in children and adults. We're proud of the approval of ERVEBO for the prevention of disease caused by Zaire ebolavirus in children as young as 12 months old, which is another milestone in our continued commitment to help address the global health threat caused by Zaire ebolavirus," said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release on August 3, 2023.
The vaccine's effectiveness when administered concurrently with antiviral medication, immune globulin, and/or blood or plasma transfusions is unknown, and the duration of protection conferred by ERVEBO is unknown.
ERVEBO includes a contraindication for individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any vaccine component, including rice protein.
The initial Ebola virus disease case first appeared in 1976 in Africa. Since then, numerous outbreaks of Zaire and Sudan have been confirmed.
As of August 3, 2023, the FDA and European Medicines Agency have approved other ebola prevention and treatment products.

The Janssen Pharmaceutical Companies of Johnson & Johnson recently announced the submission of a supplemental New Drug Application to the U.S. Food and Drug Administration (FDA) seeking to expand the indication of EDURANT® to include the treatment of human immunodeficiency virus type 1 (HIV-1) infection in children weighing 10 kg or more.
As of July 28, 2023, a parallel Marketing Authorization application was submitted to the European Medicines Agency to support a type II variation and line extension for expanded pediatric use in Europe.
If the new applications are approved, EDURANT could be administered to younger pediatric patients via standard 25 mg tablets or new 2.5 mg tablets for oral dispersion that were developed to aid administration and weight-adjusted dosing for children.
“We’ve been working to fight HIV for decades and are proud to have helped bring forward nine medicines for people living with HIV,” said Penny Heaton, M.D., Global Therapeutic Area Head, Infectious Diseases and Vaccines, Janssen Research & Development, LLC, in a related press release.
“These filings are the latest example of our longstanding work to make different treatment options available to meet the diverse needs of people living with HIV.”
EDURANT is not a preventive vaccine but is an HIV-1 specific, nonnucleoside reverse transcriptase inhibitor indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-naïve patients 12 years of age and older and weighing at least 35 kg with HIV-1 RNA less than or equal to 100,000 copies/mL.
As of August 2, 2023, the FDA has not approved an HIV vaccine.


