Search API
The World Health Organization (WHO) today reported a steady increase in the proportion of SARS-CoV-2 coronavirus variant EG.5 reported, and based on its characteristics, EG.5 may spread globally & cause a surge in COVID-19 cases.
However, as of August 9, 2023, while EG.5 has shown increased prevalence, growth advantage, and immune escape properties, there have been no reported changes in COVID-19 severity to date.
Based on the available evidence, the public health risk posed by EG.5 is evaluated as low globally, aligning with the risk associated with XBB.1.16 and the other currently circulating variants of interest.
During epidemiological week #29 (17 to July 23, 2023), the global prevalence of EG.5 was 17.4%.
This is a notable rise from the data reported four weeks prior (week 25, 19 to June 25, 2023), when the global prevalence was 7.6%, wrote the WHO.

The U.K. Health Security Agency (UKHSA) today announced the Joint Committee on Vaccination and Immunisation (JCVI) published its new advice regarding persons eligible for COVID-19 booster vaccination in autumn 2023.
Announced on August 8, 2023, the JCVI considers it appropriate to offer COVID-19 vaccines to all adults aged 65 years and over.
From autumn 2023, JCVI additionally advises that a primary course COVID-19 vaccination for persons who have not had any COVID-19 vaccines before should consist of a single dose.
Professor Wei Shen Lim, Chair of COVID-19 immunization on the JCVI, said in a press release, "The autumn booster program will continue to focus on those at greatest risk of getting seriously ill."
"It is important that everyone eligible takes up a booster this autumn – helping to prevent them from hospitalizations and deaths arising from the virus over the winter months."
Specifically, the JCVI published the following sub-groups:
- residents in a care home for older adults
- all adults aged 65 years and over
- persons aged six months to 64 years in a clinical risk group, as laid out in the Immunisation Green Book, COVID-19 chapter (Green Book)
- frontline health and social care workers
- persons aged 12 to 64 years who are household contacts (as defined in the Green Book) of people with immunosuppression
- persons aged 16 to 64 years who are carers (as defined in the Green Book) and staff working in care homes for older adults.
Dr. Mary Ramsay, Director of Public Health Programmes at the UKHSA, commented, #The booster is being offered to those at higher risk of severe illness, and by taking up the booster vaccine this autumn, you will increase your protection ahead of winter, when respiratory viruses are typically at their peak."
To optimize protection over the winter months, the JCVI advises that the autumn program should aim to complete vaccinations by early December 2023, mindful that vaccine protection is highest in the first 3 months following vaccination.
COVID-19 vaccine effectiveness rates in the U.K. are posted at this link.

Novavax, Inc. today announced its operational highlights for the second quarter ended June 30, 2023.
"During the first half of 2023, Novavax has been focused on execution, making significant progress on all three of our key priorities. We have initiated the filing for authorization of our updated XBB COVID vaccine in the U.S., with submissions in the European Union and Canada to follow. We are manufacturing at commercial scale in support of our plan to deliver our vaccine on time for the fall season," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on August 8, 2023.
"We continue to work towards deriving additional value from our pipeline and technology and will be advancing our COVID-Influenza Combination vaccine candidate through the next stage gates and towards late-stage development."
Novavax also received first Full Marketing Authorization in Europe for Nuvaxovid™ as a primary series in individuals aged 12 and older and as a booster in adults aged 18 and older.
Since authorization, Nuvaxovid™, a protein-based vaccine, has been distributed in about 40 markets.
On July 11, 2022, Novavax announced an agreement with the U.S. HHS to secure 3.2 million doses of Novavax's COVID-19 vaccine.
And the company initiated the rolling submission of a U.S. Biologics License Application for full approval of the Novavax COVID-19 Vaccine, Adjuvanted for adults and adolescents aged 12 through 18.

Local media today reported the People's Republic of Bangladesh's Directorate General of Health Services (DGHS) confirmed the total death toll has reached 293 during the dengue outbreak in 2023.
As of August 8, 2023, this data exceeds the previous record of 281 dengue-related deaths reported in Bangladesh during 2022.
The total dengue cases increased to over 59,000 in early August 3, with around 32,562 cases reported from the capital city of Dhaka.
According to the U.S. Centers for Disease Control and Prevention (CDC), dengue is a vaccine-preventable, vectorborne infectious disease and is endemic in about 125 countries in 2023.
In the U.S., Florida has reported both locally-acquired and travel-related dengue cases in 2023.
As of August 3, 2023, the U.S. States reported 225 dengue cases, and U.S. Territories reported 315 this year.
To alert international travelers, the CDC has recently issued Travel Health Notices regarding dengue outbreaks in the Americas, Africa/Middle East, Costa Rica, and Asia/Pacific Islands.
Worldwide, two dengue vaccines are in use, but access is limited.
GSK Canada recently announced that Arexvy (respiratory syncytial virus (RSV) vaccine - recombinant, AS01E adjuvanted) was approved in Canada for preventing lower respiratory tract disease caused by RSV in individuals 60 and older.
Arexvy's availability in Canada is expected ahead of the 2023/24 peak RSV season, which is during the winter.
Previous RSV vaccine authorizations have been issued in Europe, the USA, and the U.K.
Marni Freeman, Country Medical Director, GSK, said in a press release on August 4, 2023, "With the approval of Arexvy, we are excited to be able to offer an option to help protect the nearly 10 million Canadians aged 60 and older who are at risk of RSV disease."
"We're hopeful that with a vaccine now available for older Canadians, the virus' burden on our healthcare system will also be dramatically improved."
"We look forward to working with provincial, territorial, and national health authorities to ensure older Canadians at greatest risk of RSV infection can access the vaccine."
RSV is a common, contagious virus that affects the lungs and respiratory airways. The virus can affect all ages, but the impact of RSV in older adults is significant.
RSV is a seasonal respiratory virus generally identified first in Florida each year.

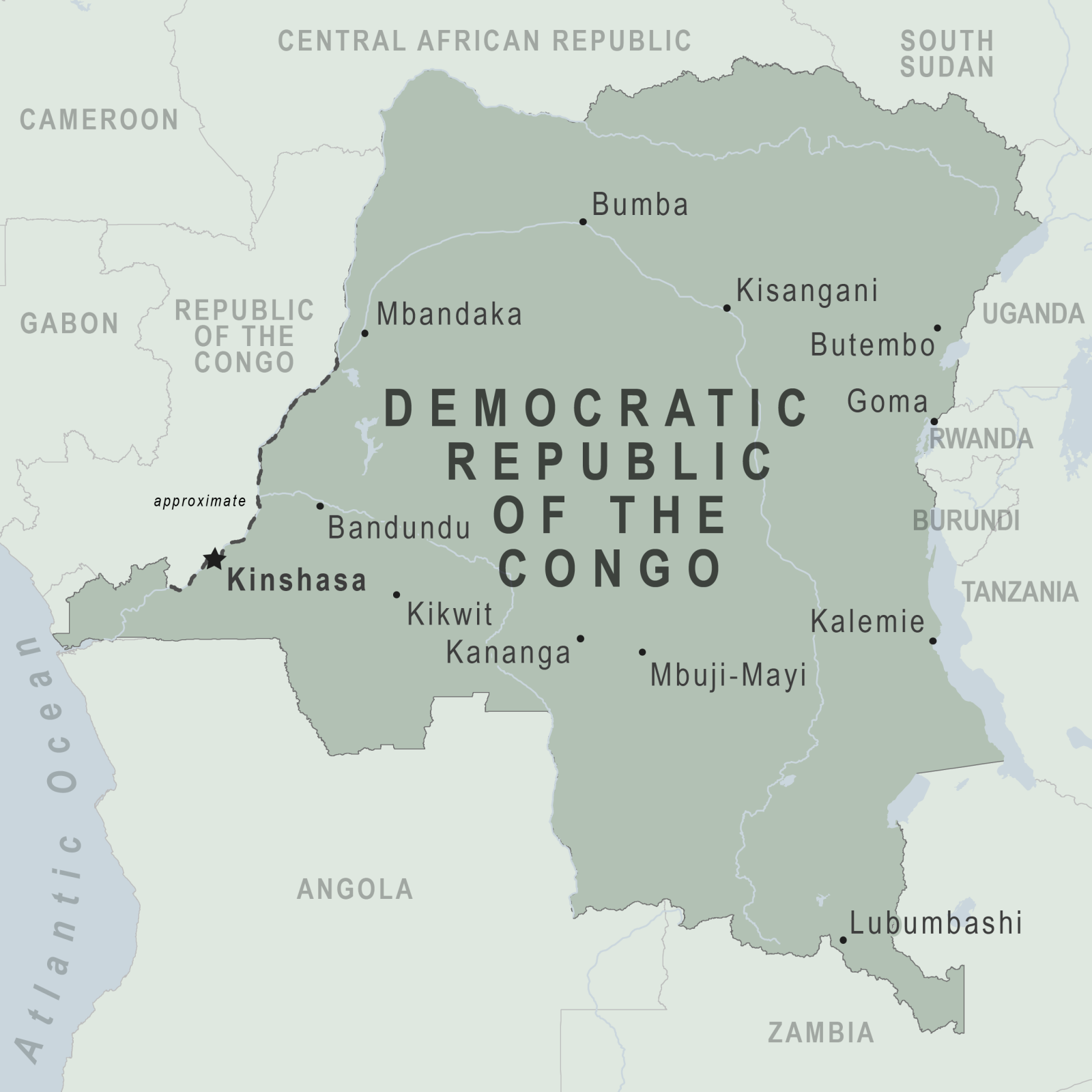
The Democratic Republic of the Congo (DRC) recently confirmed a total of 14 new polio cases.
According to the Global Polio Eradication Initiative (GPEI), there were ten circulating vaccine-derived poliovirus type 1 (cVDPV1) cases reported as of August 2, 2023.
There are now 46 polio cases in the DRC this year. Last year, there were 146 cases.
Furthermore, the DRC reported four new cases involving circulating vaccine-derived poliovirus type 2 (cVDPV2). There are now 61 cases so far this year and 367 cases reported in 2022.
Previously, the DRC launched its first significant immunization campaign in June 2023 using the novel polio vaccine type 2 (nOPV2) to protect children against polio. More than 17 million children under five in 20 provinces were included.
To alert international travelers of this health risk, the U.S. CDC included the DRC in its Level 2 - Practice Enhanced Precautions, Global Polio Travel Health Notice, which was issued on July 28, 2023.
The CDC encourages polio vaccinations for most people visiting Africa.
Previously, the U.S. Department of State issued a Level 3 advisory on July 31, 2023, saying people should reconsider travel to the DRC due to civil unrest and not visit certain providences.
Anixa Biosciences, Inc. today announced that its partner, Cleveland Clinic, has begun enrolling subjects in a treatment arm evaluating the combination of the Company's breast cancer vaccine candidate with Keytruda®.
This treatment arm aims to determine if the vaccine/Keytruda combination increases immune response.
Anixa's breast cancer vaccine is designed to generate T cells that target triple-negative breast cancer ("TNBC"). This vaccine takes advantage of endogenously produced proteins that function at certain times in life but then become "retired" and disappear from the body.
Dr. Amit Kumar, Chairman and CEO of Anixa, stated in a press release on August 7, 2023, "Cleveland Clinic has demonstrated in both preclinical and clinical studies that our breast cancer vaccine induces an immune response–including, we believe, production of T cells that can target TNBC–so we believe that the addition of Keytruda could have a synergistic effect."
"If a vaccine induces the creation of T cells targeting TNBC, and Keytruda generally maintains T cell activity, the combination could be very potent."
Keytruda, a type of immunotherapy known as a checkpoint inhibitor marketed by Merck, is approved for use with chemotherapy before surgery and then alone after surgery to treat high-risk early-stage and advanced TNBC.
The Phase 1a study evaluates the vaccine's safety, identifies the Maximum Tolerated Dose, and monitors the immune response in vaccinated women.
According to the Company, one in eight women in the U.S. will be diagnosed with invasive breast cancer at some point in their lives.

Bavarian Nordic A/S today announced positive topline results from a Phase 3 clinical trial of its virus-like particle (VLP)-based chikungunya virus vaccine candidate, CHIKV VLP (PXVX0317).
The results up to day 22 post-vaccination showed that CHIKV VLP was highly immunogenic in healthy adolescents and adults, as demonstrated by the strong induction of chikungunya-neutralizing antibodies in 98% of vaccinees in the active group.
The strong neutralizing antibody titres were equal to or exceeded the threshold agreed upon with authorities as a seroprotection marker, meeting the study's primary objectives.
Importantly, CHIKV VLP induced significant neutralizing antibodies in 97% of the subjects at two weeks post vaccination, confirming a rapid onset of protective immunity levels.
These responses were robust and durable, as 86% of the subjects had seroprotective levels of neutralizing antibodies six months post vaccination.
"We are highly encouraged by the positive topline results now demonstrated in both Phase 3 studies of our chikungunya vaccine candidate. Our focus remains to finalize the studies and prepare for regulatory submissions next year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on August 6, 2023.
"With a fast and durable response, our vaccine has the potential to be the best in class to prevent chikungunya infections in adolescents to elderly adults."
"Chikungunya can often result in a severe and incapacitating disease affects large parts of the world, and with international travel on the rise again, our CHIKV vaccine offers a significant opportunity to address this large unmet medical need."
These results help form the basis for submission of a Biologics License Application to the U.S. Food and Drug Administration and a Marketing Authorization Application to the European Medicines Agency in 2024 to support potential launch of the vaccine in 2025.
The U.S. CDC published a Morbidity and Mortality Weekly Report (MMWR) that concluded post-authorization safety data after receipt of a primary Novavax COVID-19 vaccine dose is limited by the low number of doses administered (0.01% of total COVID-19 vaccines administered), available data are consistent with those from preauthorization clinical trials.
And no new safety concerns were identified, wrote the CDC on August 4, 2023.
This MMWR stated from July 13, 2022–March 13, 2023, a total of 69,227 Novavax doses were administered to persons in the U.S., and 230 reports of adverse events after vaccination were received by the Vaccine Adverse Event Reporting System (VAERS).
Among the 230 reports received, 19 (8.3%) were classified as serious, and no deaths were reported after vaccination.
Serious reports included one case of thrombosis, two of pericarditis, one of Guillain-Barré syndrome, and two of seizure.
The remaining serious reports described chest pain, arrhythmia, sickness, hospitalization, adverse event not otherwise specified, balance disorder, peripheral neuropathy aggravated, and vaccine failure.
Limitations of this analysis include reporting biases and inconsistency in the quality and completeness of reports to VAERS.
Furthermore, VAERS data generally cannot be used to determine whether a vaccine caused an adverse event.
In addition, approximately one-half of the reports representing adverse events of special interest lacked medical records for CDC review.
Novavax COVID-19 Vaccine Adjuvanted (Nuvaxovid™, CovoVax™, NVX-CoV2373) was the first protein-based vaccine engineered from the genetic sequence of the SARS-CoV-2 beta coronavirus.

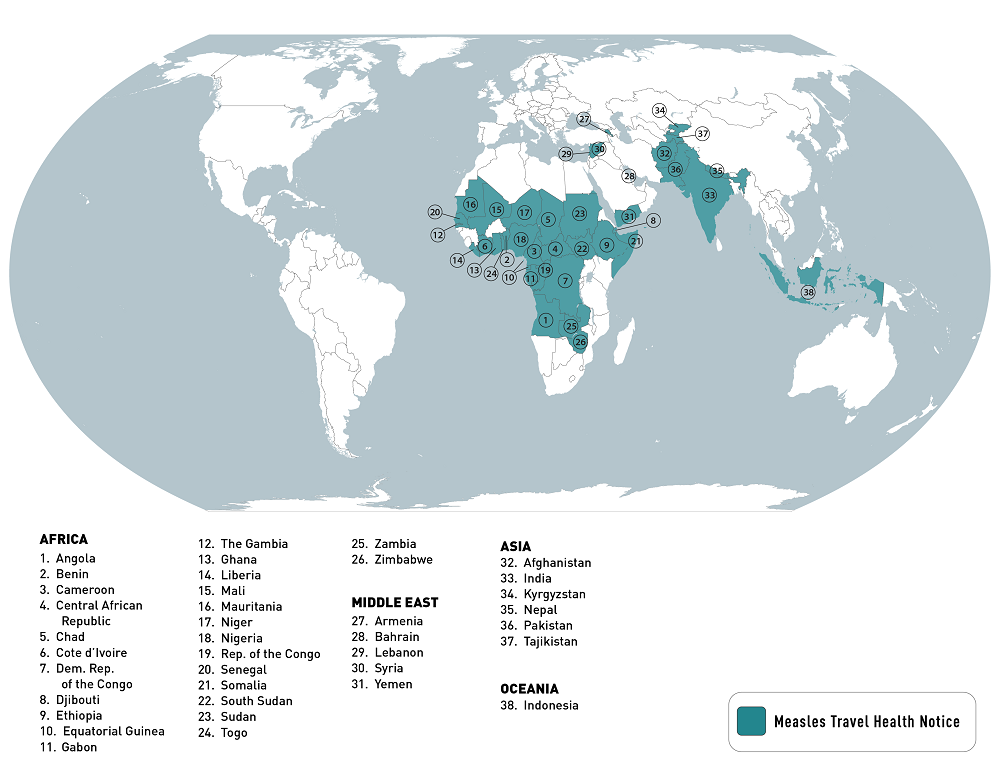
According to the U.S. Centers for Disease Control and Prevention (CDC), measles is an ongoing global health risk.
As of early July 2023, health officials in 38 countries reported large measles outbreaks.
The top ten measles outbreaks were led by India, with 67,592 cases, as of July 18, 2023.
The CDC reissued a Level 1, Practice Usual Precautions Travel Health Advisory to alert international travelers of this risk. The CDC says measles is a risk if a person is not fully vaccinated two weeks before departure or has not had measles in the past.
The CDC says international travelers, including infants 6–11 months of age and preschool-aged children, should be fully vaccinated against measles with a measles-mumps-rubella (MMR) vaccine.
This CDC web tool empowers people to determine whether or not they need additional measles vaccination before departure.
MMR vaccines are generally available in health clinics and community pharmacies in the U.S.
Measles is highly contagious, even on airplanes. Travelers should seek medical care if they develop a rash, high fever, cough, runny nose, or red, watery eyes. Vitamin A deficiency is a recognized risk factor for severe measles infections.
Travelers with suspected measles should notify the healthcare facility before visiting so staff can implement precautions to prevent the spread within the facility.
In the U.S., the CDC has reported 19 measles cases in thirteen U.S. jurisdictions as of August 3, 2023. In 2022, there were 121 measles cases.