Search API
Valneva SE today reported positive initial Phase 3 safety data in adolescents for its single-dose chikungunya virus (CHIKV) vaccine candidate VLA1553.
On August 28, 2023, Valneva announced the initial safety data generated in the ongoing trial VLA1553-321, Valneva's first clinical trial in an endemic area (Brazil) and with individuals previously infected with CHIKV, showed that VLA1553 was generally safe and well tolerated in adolescents aged 12 to 17 years, regardless of previous CHIKV infection.
In this study, 754 individuals were vaccinated in trial VLA1553-321, and the present analysis includes safety data up to Day 29. An independent DSMB has continuously evaluated safety data during the trial and identified no safety concerns.
Overall, the adverse event profile is consistent with the profile observed in Valneva's pivotal Phase 3 trial in adults, reported in March 2022.
Immunogenicity data for this new trial are expected in November 2023.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, "These new safety data in a younger population and individuals previously infected with the chikungunya virus confirm the safety profile we previously observed in adults and the elderly."
"Chikungunya represents a major threat for people traveling to or living in areas where chikungunya virus is endemic, it is, therefore, our objective to make this vaccine available to all age groups, especially as no vaccine or specific treatments are currently available for this debilitating disease."
Funded by the Coalition for Epidemic Preparedness Innovations and conducted in collaboration with Brazil's Instituto Butantan, the VLA1553-321 adolescent trial is intended to support label extension in this age group following a potential initial regulatory approval in adults from the U.S. Food and Drug Administration. The trial is also expected to support the vaccine's licensure in Brazil, which would be the first potential approval for use in endemic populations.
The present safety analysis will also enable regulatory submission to the European Medicines Agency in 2023.
If licensed, this would be the first CHIKV vaccine candidate approved.

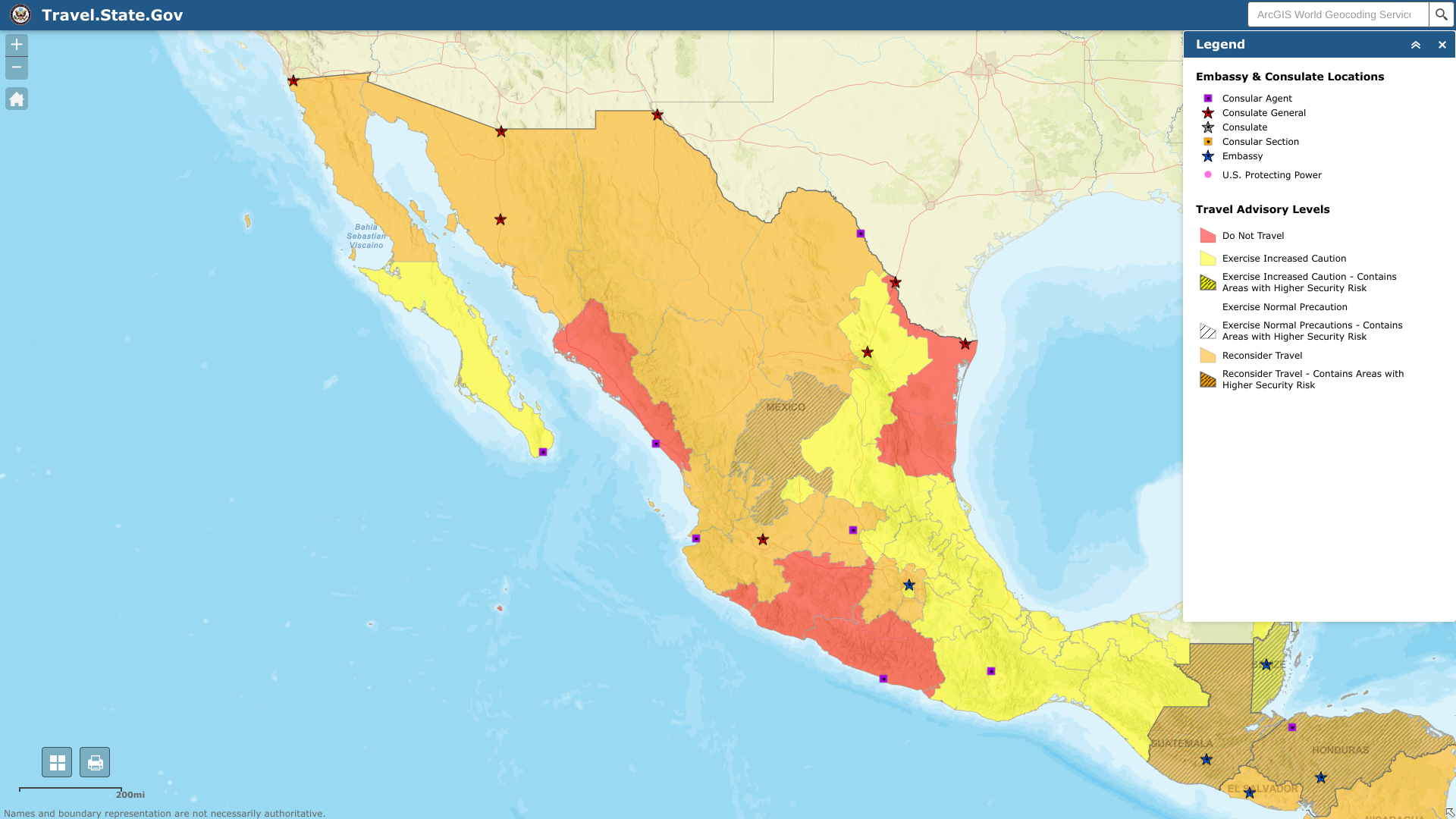
The U.S. Department of State reissued its unclassified travel advisory regarding extensive civil unrest throughout the United States of Mexico.
On August 22, 2023, the State Department reported it has limited ability to provide emergency services to U.S. citizens in many areas of Mexico, as travel by U.S. government employees to certain areas is prohibited or restricted.
In many Mexican states, local emergency services are limited outside the state capital or major cities, including Cancun.
Furthermore, U.S. government employees may not travel between cities after dark, may not hail taxis on the street, and must rely on dispatched vehicles, including app-based services like Uber and regulated taxi stands.
U.S. government employees should avoid traveling alone, especially in remote areas. U.S. government employees may not drive from the U.S.-Mexico border to or from the interior parts of Mexico, except daytime travel within Baja California and between Nogales and Hermosillo on Mexican Federal Highway 15D, and between Nuevo Laredo and Monterrey on Highway 85D.
From a health perspective, several disease outbreaks have been reported in 2023, and the U.S. CDC suggests various pre-trip vaccinations.
Since dengue outbreaks cause about 400 million infections yearly, the Pan American Health Organization (PAHO) and other agencies continue issuing health alerts for this deadly disease.
In the Americas, the total number of dengue cases reported was 2,997,097 through July 2023. Additionally, 1,302 deaths were reported in the Region.
To limit these deaths, two dengue vaccines have been approved and deployed in 2023.
According to a new study published in the journal Vaccines on August 22, 2023, the effectiveness of these vaccines varies.
Dengvaxia® has shown an efficacy of 60.8 % (95 % CI, 52.0–68.0) against symptomatic, virologically confirmed dengue (VCD) caused by any of dengue's four serotypes more than 28 days after the third dose in Asia (2 to 14-year children), and Latin America (9 to 16-year children) [15], respectively.
While QDENGA® has shown around 80.2 % (95 % CI, 73.3–85.3) efficacy.
And during a phase 1 clinical trial, the efficacy against VCD at 28 days after a single dose of a U.S. NIH-developed vaccine candidate was 79.6 %.
This study assessed the safety and immune response regarding nAbs induced by the SII Dengue vaccine in healthy adults in Australia.
The study showed the vaccine was safe and highly immunogenic in adults, primarily seronegative at baseline.
In the vaccine group, 59.0 % of participants showed DENV vaccine viremia post-vaccination.
This vaccine (Dengusiil) was formulated at Serum Institute of India Pvt. Ltd. (SII) to contain DENV 1, DENV 3, and DENV 4 serotypes at not less than 2.5 log10 PFUs and DENV 2 at not less than 3 log10 PFUs per single dose of 0.5 mL.
SII executive director Rajeev Dhere recently informed the TOI, "The Phase-I trial was conducted in Australia as we needed participants who had not been exposed to dengue previously."
"India has a significant proportion of people with dengue antibodies, so it was essential to test the vaccine's safety and effectiveness on individuals who were not already immune to the disease."
As of August 27, 2023, Dengvaxia is approved in the U.S., but availability requires pre-admission testing.
During 2023, the state of Florida and Puerto Rico have reported locally acquired and travel-related dengue cases.

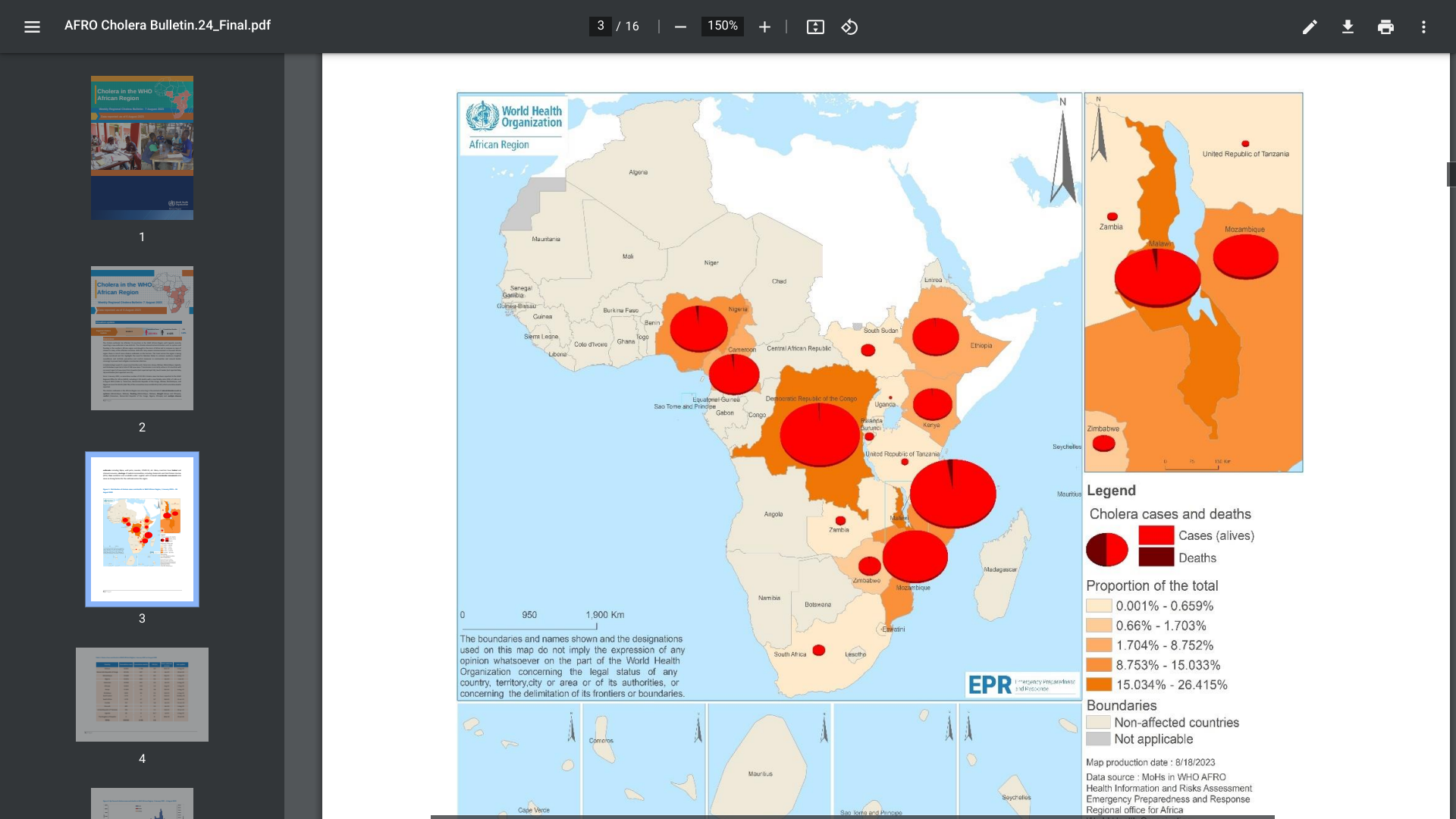
The ongoing, multi-country cholera outbreak was recently confirmed to impact African countries significantly.
The World Health Organization (WHO) Africa Region reported for Epidemiologic Week #31, seven African countries, Burundi, Cameroon, Kenya, Malawi, Mozambique, Uganda, and Zimbabwe reported 328 new cholera cases.
Cholera can kill people within hours when not treated, but immediate access to treatment saves lives.
Since January 2022, a cumulative number of 223,951 cholera cases has been reported to the WHO, including 4,125 deaths, with a case-fatality ratio of 1.8% as of early August 2023.
Based on the large number of cholera outbreaks and their geographic expansion, as well as a lack of oral cholera vaccines and other resources, the WHO continues to assess the risk at the global level as very high, as most cases are not reported.
The U.S. CDC previously confirmed an unprecedented global increase in cholera outbreaks in 2022 and 2023.
Nearly all cholera cases reported in the U.S. are acquired during international travel. The CDC's Clinician Outreach and Communication alert in 2023 identified Cholera as an acute intestinal infection that spreads through food and water contaminated with the bacterium Vibrio cholerae, often from feces. With safe water and sanitation, Cholera can be prevented.
As of August 27, 2023, access to cholera vaccines is constrained globally.
Pfizer Inc. recently announced in a press release that the European Commission (EC) has granted marketing authorization for ABRYSVO™, the company's bivalent respiratory syncytial virus (RSV) prefusion F (RSVpreF) vaccine, to help protect both infants through maternal immunization and older adults.
ABRYSVO is the first licensed vaccine designed and studied explicitly for maternal immunization. Now, a single dose of the vaccine could be administered in the EU between weeks 24 and 36 of gestation.
In addition, ABRYSVO has been studied in adults 60 and older.
"The approval of ABRYSVO in Europe marks significant progress in the scientific community's efforts to provide meaningful protection against RSV, a common respiratory virus that could potentially be severe and even life-threatening, especially for infants and older adults," commented Annaliesa Anderson, Ph.D., Senior Vice President and Head Vaccine Research and Development, Pfizer on August 24, 2023.
"Last year's significant number of newborns, children, and adults being hospitalized across Europe demonstrated the immense need for protection against severe RSV cases. The approval of the vaccine for both older adults and infants through maternal immunization is a triumph for public health, and we hope to see a tremendous impact for future (RSV) seasons."
This authorization is valid in all 27 EU member states, plus Iceland, Liechtenstein, and Norway.
RSV is a contagious virus and a common cause of respiratory illness worldwide.
In the EU, approximately 245,000 yearly hospital admissions were associated with RSV in children younger than five.
The disease burden for older adults is also significant. Each year, the virus causes more than 270,000 hospitalizations and about 20,000 deaths in individuals and older.
The virus can affect an infected individual's lungs and breathing passages, potentially causing severe illness or death.
As of August 26, 2023, two RSV vaccines are approved for seniors and available at clinics and pharmacies in the United States.

The U.S. CDC today published a Morbidity and Mortality Weekly Report (MMWR) that indicates, for the first time in ten years, human papillomavirus (HPV) vaccination initiations did not increase among adolescents in the United States.
Furthermore, coverage with one or more HPV vaccine dose among Medicaid beneficiaries declined by 3.3% in 2022 compared with coverage in 2021.
The cross-sectional analysis published on August 25, 2023, showed that HPV vaccination remained lowest among the uninsured (two of the four groups that constitute the Vaccines for Children (VFC) eligible population.
And the CDC disclosed that VFC vaccine ordering data provide additional evidence that HPV vaccination coverage might continue to decline in VFC-eligible populations.
VFC provider orders for HPV vaccines decreased 24% in 2020, 9% in 2021, and 12% during 2022 compared with 2019.
However, orders for non-HPV vaccines have rebounded to prepandemic levels (Whitlatch F, CDC unpublished data, 2023).
The HPV vaccine is the most expensive of all routinely recommended adolescent vaccines. And reimbursement levels for costs by private payers are adequate, but return margins are small for nonpediatric specialties, according to a study published in Annals of Family Medicine in July 2023.
In the U.S., the CDC's Advisory Committee on Immunization Practices recommends that children aged 11–12 years receive HPV vaccines, and some children can be started at age 9.
The CDC says HPV vaccination is the most effective way of preventing cervical cancer in women and other sexually transmitted HPV cancers.
Various HPV vaccines are available at most health clinics and pharmacies in the U.S.
This CDC report used two analyses of 2022 NIS-Teen data to examine vaccination coverage among U.S. adolescents 13 to 17 years of age.

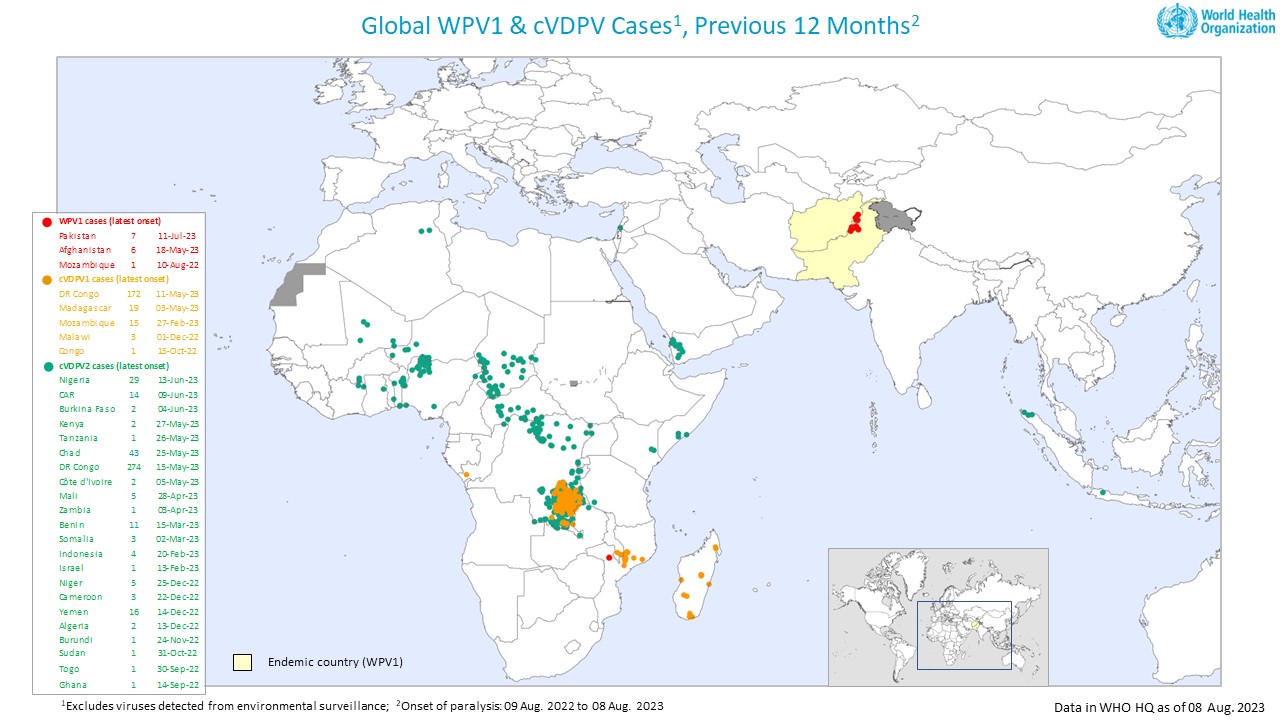
The World Health Organization (WHO) today announced that although encouraged by the reported progress, the Committee unanimously agreed that the risk of international spread of poliovirus remains a Public Health Emergency of International Concern (PHEIC) and recommended the extension of Temporary Recommendations for a further three months.
As of August 25, 2023, only two genetic clusters of WPV1 were identified, compared to three in 2022 and five in 2021.
However, there have been multiple chains of transmission within these two genetic clusters, detected primarily in the endemic zones of Eastern Afghanistan and South KP of Pakistan, including an extreme orphan virus, indicating some gaps in surveillance.
And there is a large pool of unvaccinated "zero dose" children in Afghanistan, which could reintroduce wild-type poliovirus into the southern region.
The also noted suboptimal immunization coverage during campaigns in southeastern Africa, Malawi, Mozambique, Zambia, and Zimbabwe. The nOPV2 vaccine has been deployed over 700 million times in Africa.
Based on the current situation and the reports provided by affected countries, the WHO Director-General accepted the Committee's assessment. It determined that the poliovirus situation continues to constitute a PHEIC.
While poliovirus has been detected in southern New York in 2022 and 2023, no outbreak has been confirmed.
Various polio vaccines are approved and available in August 2023 in the U.S. and globally.
Since the global shortage of oral cholera vaccines (OCV) is forecast to continue until 2025, including in the United States, two companies today announced they are taking action to increase supply.
On August 25, 2023, GC Biopharma confirmed that it signed an MOU with Eubiologics for a co-production of Euvichol®, a World Health Organization (WHO) certified vaccine.
The two companies will coproduce Eubiologics in the first half of 2024 to supply to UNICEF, which has requested an additional supply to cope with the recent spread of cholera infection in many regions, including Africa.
Kyeong-Ho Min, Vice President of Eubiologics, commented in a press release, "With the more frequent floods and droughts due to climate change and global warming, the world is currently experiencing rapid spread of cholera, leading to a shortage of vaccine supply."
Under the MOU, Eubiologics, a developer and producer of Euvichol, takes charge of the bulk vaccine production, and GC Biopharma will be in control of the packaging process, including vial bottling.
Euvichol is an OCV jointly developed by Eubiologics and the International Vaccine Institute that obtained WHO Prequalification in 2015.
Since supplying to UNICEF in 2016, the cumulative supply has exceeded 100 million vaccine doses. Eubiologics is currently providing 100% of cholera vaccines administered by UNICEF.
During 2023, about 49 million OCV doses have been requested, of which 39% were approved for 11 countries, according to WHO report #5.
The WHO stated in August 2023, the current cholera epidemic has deteriorated. Therefore, the WHO assessed the risk of cholera outbreaks at the global level as very high.

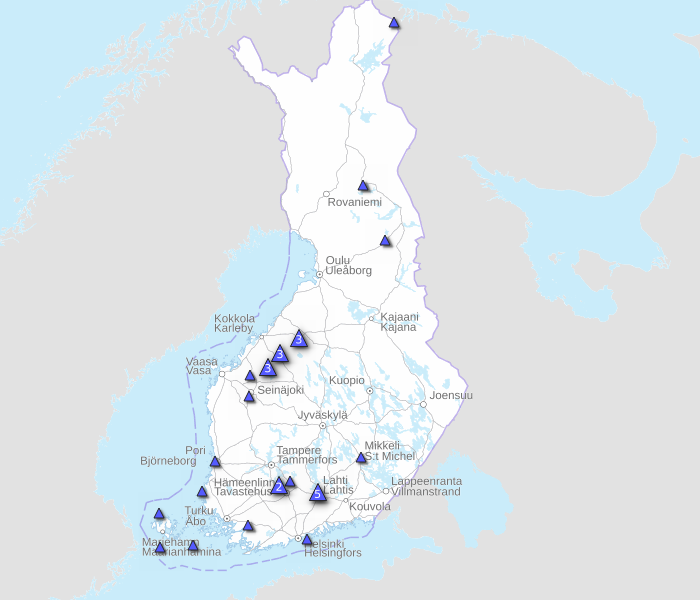
The Republic of Finland recently reported another H5N1 avian influenza outbreak at a fur farm involving blue foxes. This outbreak, reported by EuroNews on August 24, 2023, increases the number of 'bird flu' outbreaks at such farms to 23.
In June 2023, several outbreaks of H5N1 were identified among larids in Finland. The first cases in fur farms were detected in July.
Given these findings, the Finnish authorities decided to cull 120,000 foxes and mink on farms affected by the epidemic.
Finland is Europe's leading producer of fox fur, with around 400 fur farms. For example, FashionFinFur is owned by a fur-farming family in Vörå, Finland, that has been In the business since the 1980s.
In the northern hemisphere, bird flu outbreaks among birds, mammals, and people continue in 2023.
As of August 2023, access to bird flu vaccines is controlled by government agencies.