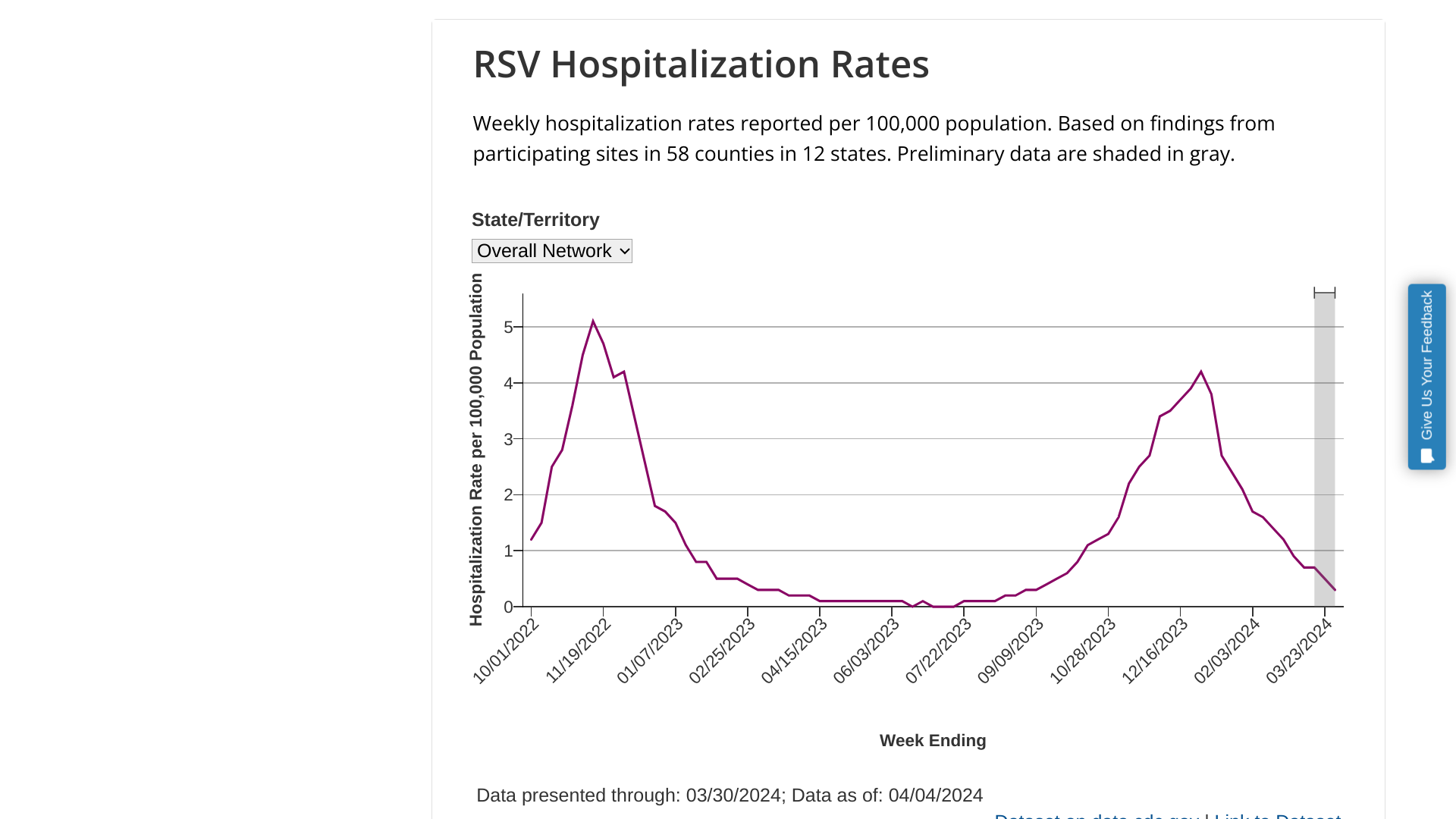
Uromune MV140 Urinary Tract Infection (UTI) Vaccine
Uromune MV140 Recurrent Urinary Tract Infection (rUTI) Vaccine Clinical Trials, Dosage, Indication, Side Effects
Immunotek S.L. Uromune™ MV140 is an inactivated, oral spray, novel sublingual mucosal-based bacterial vaccine that reduces the recurrence of Urinary Tract Infections (rUTI). Since 2017, research has shown that MV140 induces systemic and genitourinary tract immune responses, particularly in the bladder's innate immune system. MV140 contains four whole-cell inactivated bacteria, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, and Enterococcus faecalis, in a suspension with water. Uropathogenic Escherichia coli (UPEC) is responsible for the majority of community-acquired urinary tract infections (UTIs).
Uromune became available for clinical evaluation in 2010. In July 2012, an observational study described its use in the clinical literature, favoring it as an effective strategy to reduce the frequency, duration, severity, and costs of rUTIs. A retrospective study conducted by the Royal Berkshire Hospital, along with a subsequent international randomized controlled trial that followed patients for 12 months, was published in BJU International in November 2017. In August 2020, CAUJ published a systematic review (Vol. 14, No. 8) of the current literature and plans regarding the use of MV140. Five studies met the inclusion criteria for primary review. In two retrospective comparative studies, subjects treated with Uromune daily for three months (519 women in total) had significantly higher UTI-free rates (35–90%) than subjects treated with six months of antibiotic prophylaxis (0% in 499 women in total) over 15 months (p<0.001 for both studies). In three prospective, uncontrolled studies, UTI-free rates for subjects treated with Uromune ranged from 33% to 78% over 9 to 24 months.
In a multicenter, randomized, double-blind, placebo-controlled, parallel-group 1-year clinical trial of modest size and duration, funded by Inmunotek S.L. and Syner-Med Ltd, published in January 2022 by NEJM Evidence, MV140, a sublingual preparation of whole-cell inactivated bacteria, showed promising clinical efficacy in reducing recurrent UTI in women suffering from this condition (free of UTIs, 56% (95% CI, 44% to 67%) and 58% (95% CI, 44% to 67%) of women who received 3 and 6 months of MV140 treatment). Adverse effects were not clinically limiting.
On April 22, 2024, the findings from a small study suggest that 54% of participants who were given MV140 remained UTI-free for up to nine years after treatment, with no notable side effects. On December 18, 2024, a prospective real-life study found that in the 12 months after MV140 treatment outset, 38% of patients were UTI-free, 34% reported 1 or 2 UTI episodes, and the remaining 28% presented three or more UTIs, corresponding to a mean reduction of 3.20 (2.87–3.53, 95% C.I.; p < 0.001) UTI episodes per year per patient. The effectiveness of MV140 was the same regardless of sex, BMI, regular sexual activity, hypertension, diabetes mellitus, depression, paraplegia, performance of intermittent self-catheterization, indwelling bladder catheter, or previous use of other UTI-preventing vaccines. A higher effectiveness was observed in postmenopausal women compared to premenopausal women (74.7% vs. 59.4%, respectively, p = 0.029). And 73% of patients reported a reduction in symptom severity or the number of disease days, with a mean global satisfaction score of 7.52/10.
Immunotek S.L. is at 5 Punto Mobi Street, 28805 Alcalá de Henares, Madrid, Spain. In 2019, Immunotek launched an Expanded Access Program for UROMUNE for individuals who had failed antibiotic therapy. UTI vaccines are conducting clinical trials and accepting new participants.
Uromune MV140 rUTI Vaccine Availability 2025
In 2025, the oral spray Uromune vaccine is commercially available in approximately 20 countries through Expanded Access Programs. Since 2017 (NCT04173013), about 21,000 patients, representing 1.5 million doses of Uromune, have been administered.
In England, vaccination clinics in Hampstead, London, and the Marylebone area offer Uromune vaccine appointments, which are available at this link. In 2014, the first patients in Reading received the novel oral spray-based vaccine MV140 (Uromune). About 54% of the initial participants assessed were UTI-free for up to nine years after treatment, with an average UTI-free period of 4.5 years, and suffered no notable side effects. Using Hospital Episode Statistics data for 2023 to 2024, 189,756 hospital admissions related to UTI occurred in England, resulting in a total of 1.2 million NHS bed‑days; this amounted to an average of 6 bed-days per infection.
As of December 2025, Uromune is unavailable in the United States, Canada, and Spain.
Uromune MV140 rUTI Vaccine Efficacy
Several studies have evaluated the beneficial role of MV140 in preventing recurrent urinary tract infections (rUTIs). In 2012, an Original Article concluded that the results of the study favored the use of this bacterial-based therapeutic vaccine to reduce the frequency, duration, severity, and costs of rUTIs. In 2017, a prospective study suggested that Uromune was safe and effective at preventing UTIs in women. The journal Vaccine published a 2021 prospective analysis of MV140 in older people from nursing homes. A total of 200 subjects (160 females, with a mean age of 82.67 years; 40 males, with a mean age of 80.23 years) had a median of 4 UTI (or asymptomatic bacteriuria) episodes per month, treated with antibiotics. The UTI rate decreased in females following MV140 treatment to a median of 0.1 UTIs per month. U I-free rates for the 12 months following initiation of the vaccine were observed in 18% of women, while 81.7% were treated for fewer than 3 UTIs per year.
The NEJM Evidence published an Original Article on January 21, 2022: Sublingual MV140 for Prevention of Recurrent Urinary Tract Infections. In this Phase 3 controlled clinical trial of modest size and duration, MV140 demonstrated promising clinical efficacy in reducing the rUTIs in women.
Ontario, Canada-based Dr. Curtis Nickel presented the pivotal randomized, placebo-controlled trial. In women with rUTI, MV140 significantly reduced the risk of UTI, from a median UTI rate of 3 in the placebo group over 9 months to 0 in both the 3- and 6-month groups. The vaccine decreased the overall need for antibiotics, healthcare resources, and costs associated with rUTI management.
A study published in 2023 reported that, based on observational, prospective, and randomized placebo-controlled studies, MV140 has been shown to safely prevent (or reduce the risk of) UTIs, reduce antibiotic use, reduce overall management costs, and reduce patient burden while improving the overall quality of life in women suffering from rUTIs.
A prospective, descriptive, multicenter study published in February 2024 found that 1,104 women with three or more uncomplicated urinary tract infections (UTIs) who received Uromune immunoprophylaxis had better outcomes and benefited from a Uromune prophylaxis protocol. Patients with fewer than 5 UTIs at baseline were more likely to achieve better outcomes and benefit from Uromune prophylaxis. Uromine vaccination reduced the number of UTIs by about 70% and increased the time to the next UTI from 48 days to 275 days.
On March 29, 2024, a Secondary Analysis of a Randomized, Placebo-Controlled Efficacy Phase 3 Study found that three months of MV140 use are associated with a reduction in the personal burden of UTI, as evidenced by a decrease in overall UTI symptoms and antibiotic use, thereby improving the quality of life in women with recurrent UTI (rUTI).
Initial results from a long-term follow-up study of the safety and effectiveness of the MV140 vaccine for recurrent UTIs were announced at the European Association of Urology Congress on April 6, 2024. These results show that 54% of study participants, both men and women with recurrent urinary tract infections (UTIs), remained UTI-free for nine years following vaccination.
Uromune MV140 rUTI Vaccine Administration
In Phase 3 clinical research, MV-140 was administered sublingually, a method known to bypass degradation by gastric fluids and gastrointestinal enzymes. A pineapple-flavored suspension spray was administered under the tongue daily for three months. Oral self-administration is a potential alternative to antibiotic treatments.
Uromune MV140 rUTI Vaccine Mechanism of Action
MV140's mechanism of action induces antibody production and activation of human dendritic cells, generating T helper (Th) 1, Th17, and interleukin-10. This results in anti-inflammatory T-cell responses in secondary lymphoid organs and locally in the bladder.
rUTI Prevention
Royal Berkshire Foundation Trust shows that over 50% of people given the Uromune MV140 vaccine can prevent recurrent urinary tract infections for up to 9 years. A systematic review reported UTI-free rates among MV140-vaccinated women of 32–90%.
Uromune MV140 rUTI Vaccine Indication
An estimated 400 million UTIs occur each year globally, with over 200,000 associated deaths, making UTIs one of the most common bacterial diseases and drivers of antimicrobial usage. Uropathogenic Escherichia coli (UPEC) causes 80% of urinary tract infections, primarily cystitis. MV140 has demonstrated clinical efficacy in preventing recurrent cystitis. Based on observational, prospective, and randomized placebo-controlled studies, MV140 has been shown to safely avoid or reduce the risk of UTIs, decrease antibiotic use, and lower overall management costs and patient burden, while improving the overall quality of life in women suffering from recurrent UTIs (rUTIs). Early clinical data suggest that MV140 vaccination is more effective than antibiotic prophylaxis in preventing recurrent urinary tract infections (rUTIs). Current rUTI infections, defined as three or more episodes in 1 year or two or more infections in 6 months, affect approximately 10% of women.
UTIs are consistently reported as the leading site of infection in long-term care facilities. The prevalence of bacteriuria in patients without an indwelling catheter is between 25% and 50% for women and 15% and 40% for men. The risk rate of UTIs in older men is lower than in women.
Children suspected of having a urinary tract infection (UTI) are investigated and treated differently in various settings. Pediatric urinary tract infections (UTIs) account for 0.7% of physician office visits and 5-14% of emergency department visits annually. Among infants presenting with fever, the overall prevalence (95% confidence interval) of urinary tract infections (UTIs) was 7% (5.5-8.4).
Uromune MV140 rUTI Vaccine Side Effects
As of 2025, no notable side effects have been reported. Studies evaluating MV140 use in women with rUTIs have not reported any major safety concerns. The American Urological Association Education and Research, Inc., stated in May 2024 that a long-term study of Uromune® showed an excellent safety profile with minimal long-term adverse events. In February 2024, a review found that no adverse reactions were reported in the two major comparative studies comparing MV140 to antibiotics. Only 15 reports of adverse reactions (ARs) have been filed for over 1.5 million doses (data on file, Pharmacovigilance Department, Inmunotek, Spain).
Uromune MV140 rUTI Vaccine Cost
The cost of Uromune vaccination varies by country. In Australia, a three-month treatment of the Uromune MV140 Vaccine costs about $320. In Mexico, the Benavides website lists the Uromune vaccine 6ml spray for $3,882 (pesos). In most UTI vaccine clinics, additional service fees apply.
Uromune MV140 rUTI Vaccine Price Benefit
Clinical studies confirm that MV140 significantly reduces the number of UTIs when compared to prevaccination UTI rates, antibiotic prophylaxis, and placebo. Results from this observational, prospective study, including women with rUTI, demonstrated a significant clinical, healthcare, and economic impact due to their associated direct costs, primarily from medical consultations. The decreased frequency of urinary infections following vaccination with Uromune resulted in a significantly reduced need for healthcare resources. Likewise, a marked drop in healthcare-associated expenses was observed, including those driven by antibiotic use, primary care physician consultations, specialized care, and complementary tests, resulting in a significant global reduction in expenditures associated with rUTI.
Uromune MV140 Vaccine European Guidelines
The European Association of Urology published rUTI guidelines that include a systematic review of two retrospective and three prospective cohort studies, which concluded that MV140 might decrease the number of rUTI episodes and/or increase the probability of patients being UTI-free.
In November 2024, The JAMA Network published the third WikiGuidelines consensus statement, providing an evidence-based approach to UTI management developed by a global network of experts for practical use across diverse clinical settings. Despite decades of research and nearly 1,000 studies reviewed, we remain unable to provide a clear recommendation on many, if not all, essential aspects of preventing, diagnosing, and treating urinary tract infections.
Recurrent Uncomplicated UTI in Women: AUA/CUA/SUFU Guideline
Published by the American Urology Association, this document seeks to establish guidance for the evaluation and management of women with rUTI to prevent inappropriate antibiotic use, decrease the risk of antibiotic resistance, reduce adverse antibiotic use effects, provide guidance on anti-nonantibiotic-antibiotic strategies for prevention, and improve clinical outcomes and quality of life by reducing the recurrence of UTI events.
UTI Diagnostics
In January 2025, a mini-review summarized the current state of UTI diagnostics, covering existing and emerging technologies, including rapid molecular-based pathogen identification, next-generation sequencing, and advanced antimicrobial susceptibility testing.
Uromune MV140 UTI Vaccine News
January 22, 2025 - VIDEO: Have you heard of the UTI vaccine?
June 1, 2024 - Journal of Urology article: It's Time to Embrace Vaccination as We Enter the Postantibiotic Era of Recurrent Urinary Tract Infection Management.
May 2, 2024 - The journal Nature published: UTIs make life miserable — scientists are finding new ways to tackle them.
April 22, 2024 - University of Oxford News: Recurrent Urinary Tract Infections can be prevented for up to nine years longer than with standard antibiotic treatments in over half of the people treated, a new study has found.
April 6, 2024 - Gernot Bonkat, the EAU Chairman of Guidelines on Urological Infections, said: "These findings are promising. RUTIs are a substantial economic burden, and the overuse of antibiotic treatments can lead to antibiotic-resistant infections. A follow-up study revealed encouraging data about the long-term safety and effectiveness of the MV140 vaccine. Further research into more complex UTIs and looking at different patient groups is needed to optimize how to use this vaccine better."
December 7, 2023 - An Original Article: Evaluation of MV140 in preventing recurrent urinary tract infections: a multicenter double-blind randomized controlled trial protocol.
February 21, 2023 - MDPI published a study titled "An Effective Sublingual Vaccine, MV140, Safely Reduces Risk of Recurrent Urinary Tract Infection in Women." Based on observational, prospective, and randomized placebo-controlled studies, MV140 has been shown to safely prevent or reduce the risk of UTIs, decrease antibiotic use, and lower overall management costs and patient burden, while improving the overall quality of life in women suffering from recurrent UTIs (rUTIs).
January 23, 2023—The Mexican Journal of Urology published results from a clinical study on the behavior of the MV140 vaccine to prevent recurrent urinary tract infections in patients with metabolic syndrome and smoking.
January 21, 2022 - The NEJM published an ORIGINAL ARTICLE: Sublingual MV140 for Prevention of Recurrent Urinary Tract Infections. M 140 demonstrated promising clinical efficacy in reducing the recurrence of urinary tract infections (UTIs) in women affected by this condition.
September 1, 2021 - Clinical studies confirm the theoretical benefits of the MV140 vaccine as a safe and effective strategy to reduce the incidence and/or prevent rUTI in women. Physicians who treat rUTI and women who suffer from rUTI will soon have an evidence-based alternative to antibiotic management.
October 25, 2018 - This Review describes the pathogenesis of uncomplicated UTIs and non-antibiotic treatments.
November 23, 2017 - Treating women with recurrent urinary tract infections with the bacterial vaccine Uromune® in the U.K. Of the 75 women who completed treatment, 59 (78%) had no subsequent UTIs in the follow-up period.
Uromune MV-140 Vaccine Clinical Trials
As of May 2024, at least eight clinical studies of Uromune, including one phase 3 randomized controlled trial, have been conducted. Four European clinical studies investigated Uromune for the prevention of recurrent urinary tract infections (rUTIs). Over 1,400 women experienced UTI-free rates ranging from 33% to 90%.
Phase 3 study (NCT02543827) - The median (interquartile range) of UTI episodes was 3 (0.5 to 6.0) for placebo compared with 0.0 (0.0 to 1.0) in both groups receiving MV140. Among women treated with placebo, 25% (95% confidence interval [CI], 15% to 35%) were free of UTIs compared with 56% (95% CI, 44% to 67%) and 58% (95% CI, 44% to 67%) of women who received 3 and 6 months of MV140 treatment, respectively. A total of 205 adverse events (AEs) were reported in 101 participants, with 81, 76, and 48 events in the placebo, 3-month MV140, and 6-month MV140 groups, respectively. In this small, controlled trial of modest duration, MV140 demonstrated promising clinical efficacy in reducing the recurrence of UTIs in women with this condition.
Cochrane Central Register of Controlled Trials—First experience in the United Kingdom with the novel sublingual vaccine uromune®in the treatment of women with recurrent urinary tract infections, Yang B, Foley S, International Journal of Surgery (London, England), 2017, 47, S3‐S4 | added to CENTRAL: January 31, 2018 | 2018 Issue 1.
BJUI published an original article in 2017. It was the first experience in the U.K. of treating women with recurrent urinary tract infections using the bacterial vaccine Uromune®. Study Results: Of the 75 women who completed treatment, 78% experienced no subsequent UTIs during the follow-up period. Before treatment, all women had experienced a minimum of three or more episodes of rUTI during the preceding 12 months. In proportion, the majority of recurrences occurred in postmenopausal women. The patient had to stop treatment because of an adverse event (rash over the face and neck).
In 2015, a retrospective cohort study evaluated the medical records of 669 women with recurrent urinary tract infections (rUTIs) in Spain. The study participants were divided into two groups: 339 received a 6-month prophylaxis with antibiotics, and 360 received a 3-month prophylaxis with a sublingual bacterial preparation (MV 140-Uromune). The time from the prophylaxis period to the appearance of a new infection (as assessed by uroculture) was recorded and followed for 1 year. The absolute risk reduction (ARR) and number needed to treat (NNT) were also calculated. Results: All patients treated with antibiotics experienced a new urinary tract infection (UTI) during the 12-month scoring period, with a median of 19 days free of UTIs (range, 5–300 days). In the group treated with the bacterial preparation, 35 patients (9.7%) developed a urinary tract infection (UTI) during the same period. Kaplan-Meier curves comparing the cumulative survival (disease-free time) between the two groups showed a significant difference (P < 0.0001). The absolute risk reduction (ARR) was 90.28% (87.18–93.38), and the number needed to treat (NNT) was 1.1 (1.1–1.1). These results suggest that treatment with this bacterial preparation significantly reduces the incidence of rUTIs, making it an effective strategy for reducing the frequency of rUTIs. I reduce antibiotic consumption, aligning with current recommendations to address increasing antimicrobial resistance.