Search API
The World Health Organization (WHO) has issued Disease Outbreak News (DON513) after confirming Timor-Leste's first fatal human case of rabies by its Ministry of Health and Ministry of Agriculture, Fishery and Forestry.
As of late March 2024, 29 suspected rabies cases in humans exposed to dogs had been reported in Oecusse Municipality this year.
The public health response is ongoing and includes dog vaccination and ensuring the availability of rabies vaccines and human rabies immunoglobulin.
As of April 10, 2024, the available information suggests a high risk of rabies at the national level, whereas the risk at regional and global levels is low, according to the WHO's assessment.
The WHO noted that Oecusse is an enclave of Timor-Leste located within Indonesia East Nusa Tenggara province (NTT), where six human rabies deaths have been recorded.
In 2023, a total of 30 human rabies deaths were reported from NTT province.
Rabies is a vaccine-preventable, zoonotic, viral disease affecting the central nervous system. The WHO procured 1,000 doses of human rabies vaccines and distributed them to Timor-Leste hospitals and health clinics.
In the United States, various rabies vaccines have been approved.
Furthermore, a rabies vaccine candidate (PIKA Rabies Vaccine) reported very positive results from a phase 3 clinical study.

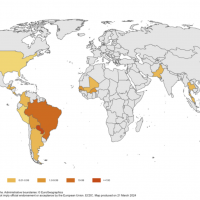
As the world battles the resurgence of measles outbreaks, most vaccination campaigns are reactive.
According to an announcement by Ginkgo Bioworks, an AI-enabled measles forecasting model may soon empower proactive public health measures, such as immunization campaigns.
Announced today, Ginkgo's epidemiological modeling experts and Northeastern University researchers were awarded a grant from the Bill & Melinda Gates Foundation to develop a forecasting model to assess the risk of measles outbreaks and inform decision-making for timely interventions.
This innovative model will draw upon traditional and non-traditional data, including public health reports, travel patterns, economic activity, and other factors, and utilize AI approaches such as machine learning and deep learning to structure and analyze a multitude of data sources to produce actionable insights.
Matt McKnight, General Manager for Biosecurity at Ginkgo Bioworks, commented in a press release on April 10, 2024, "If we wait until large pockets of measles show up in hospital systems to launch public health responses, we are missing a critical window to act and slow the spread of this debilitating and highly contagious disease."
"Modern data and AI tools can shift the biosecurity and public health paradigm from reactive to proactive by helping global health leaders make more timely, effective decisions to prevent outbreaks from happening in the first place."
Measles is a highly contagious and often severe disease that most commonly affects children. While the widespread availability of measles vaccines has dramatically reduced the disease burden over the past several decades, cases are on the rise in the U.S. in 2024.
The majority of measles cases imported into the United States occur in unvaccinated residents who become infected during international travel, says the U.S. CDC.
For example, a substantive, ongoing measles outbreak has occurred in Chicago, Illinois, over the past two months.
A next-generation rabies vaccine candidate could soon replace current options. Despite being a vaccine-preventable disease, rabies persists in over 150 countries and territories.
YS Biopharma Co., Ltd. today announced positive interim results from the ongoing Phase 3 clinical trial of its PIKA Rabies Vaccine.
The study's interim results indicate that the PIKA Rabies Vaccine has successfully met the primary endpoints of the Trial and has the potential to achieve best-in-class accelerated protection and meet the goal of a one-week rabies vaccine regimen to replace the conventional three- or four-week regimens.
The PIKA Rabies Vaccine utilizes YS Biopharma's proprietary PIKA adjuvant technology and is designed to produce a more robust immune response in an accelerated timespan than existing rabies vaccines.
The PIKA Rabies Vaccine was granted U.S. FDA orphan drug designation for prevention of rabies virus infection, including post-exposure prophylaxis for rabies.
Dr. David Shao, CEO of YS Biopharma, stated in a press release on April 9, 2024, "We remain committed to working closely with drug regulatory agencies in various countries, including the Philippines, Pakistan, Singapore, China, and other jurisdictions regarding the product registration and marketing application."
"We eagerly anticipate the early approval of this innovative therapy for the benefit of patients worldwide."
According to the World Health Organization, two types of vaccines protect people against rabies: nerve tissue and cell culture vaccines.
In the United States, rabies vaccines have been U.S. FDA-approved.
Bats are one of the most commonly reported rabid animals in the U.S. and are the leading cause of rabies deaths in people, says the U.S. CDC.
About 5,000 animal rabies cases are reported annually. Human rabies cases in the U.S. are rare, with only 1 to 3 cases reported annually.
Rabid bats have been found in all 49 continental states. Only Hawaii is rabies-free.
Worldwide, infected dogs cause approximately 59,000 rabies deaths.

Transgene and NEC Corporation today announced that new data will be presented on TG4050, an individualized neoantigen cancer vaccine, at the American Association for Cancer Research (AACR) Annual Meeting.
TG4050 is being evaluated in a randomized multicenter Phase I/II trial as a single agent in the adjuvant treatment of HPV-negative head and neck cancers.
Key findings of the poster presentation obtained in the Phase I part of the trial (NCT04183166) include, but are not limited to, the following:
All 16 patients who received TG4050 are disease-free after a median 18.6-month follow-up. Out of the 16 patients in the control observation arm, three patients have relapsed. For this head and neck cancer patient population and with the current standard of care (chemoradiotherapy), approximately 40% of patients are expected to relapse within 24 months following surgery and adjuvant therapy. Also, the tumor immune contexture, expression of immune factors, mutational burden, and tumor infiltrates are associated with challenging prognoses.
Specific cellular immune responses were detected in the 16/17 patients who received TG4050 (16 patients from the treatment arm and one from the observation arm treated after relapse) using stringent testing conditions.
TG4050 induced persistent immune responses against multiple targets in several patients. T-cell responses were maintained beyond 211 days (7 months) after the initiation of the treatment.
Dr Oliver Lantz, Head of the clinical immunology laboratory at Institut Curie, commented in the April 9, 2024, press release, "The immunological data generated by TG4050 demonstrate a robust and specific cellular immune response, even under stringent measurement criteria."
"The diversity, depth, and duration of these responses were most certainly a key factor in preventing relapse in the patients treated with TG4050."
According to statements, Transgene and NEC are preparing a randomized Phase II extension of this trial, slated to start in the second quarter of 2024.
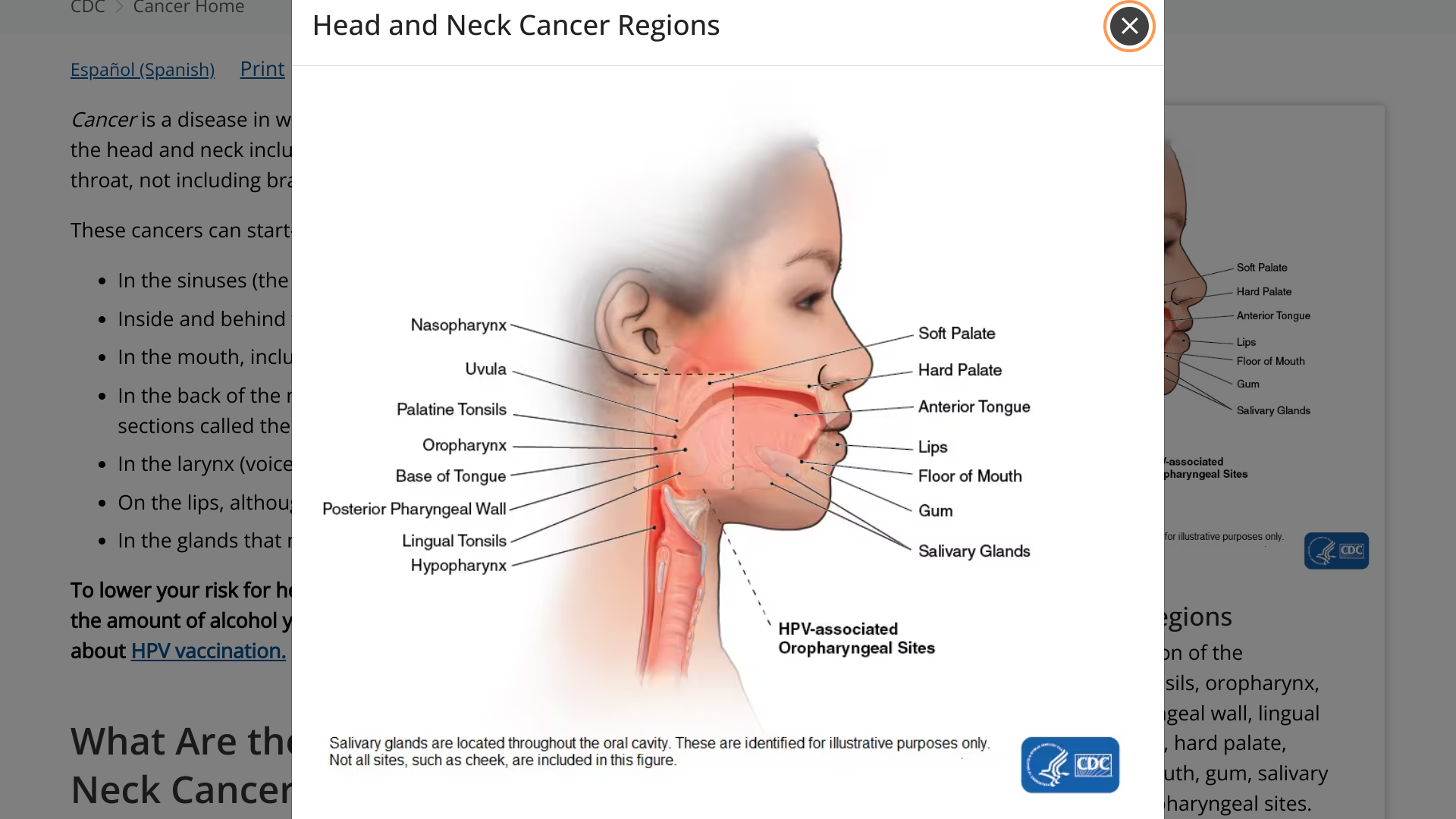
The U.S. CDC says cancers of the head and neck include cancers that start in several places in the head and throat. Cancer is a disease in which cells of the body grow out of control.
About 70% of cancers in the oropharynx (which includes the tonsils, soft palate, and base of the tongue) are linked to human papillomavirus, a common sexually transmitted virus, says the CDC.
During the recent pandemic in the United States, leading public health officials noted a shift in the attitudes of pregnant and recently pregnant women towards vaccination.
As per an Original Investigation published by The JAMA OPEN Network today, during the first waves of the SARS-CoV-2 coronavirus outbreak, 76% of pregnant women received the COVID-19 vaccination.
Study participants were about 31 years of age and enrolled in the U.S. CDC's Vaccine Safety Datalink and were asked about their vaccination status.
Response rates were 43.5% for wave 1 (652 of 1500 individuals sampled) and 39.5% for wave 2 (575 of 1456).
Overall, 76.8% (95% confidence interval, 71.5%- 82.2%) reported having received one or more COVID-19 vaccinations.
Spanish-speaking Hispanic respondents had the highest weighted proportion of respondents with one or more vaccinations.
Additionally, these women were asked if they agreed with the statement that 'COVID-19 vaccines are safe.'
These researchers wrote on April 9, 2024, that there is decreasing confidence in COVID-19 vaccine safety in diverse pregnant and recently pregnant insured populations, which is a public health concern.
This study was supported by the U.S. CDC, contract number 200-2012-53581-0011, and no industry conflicts of interest were highlighted.

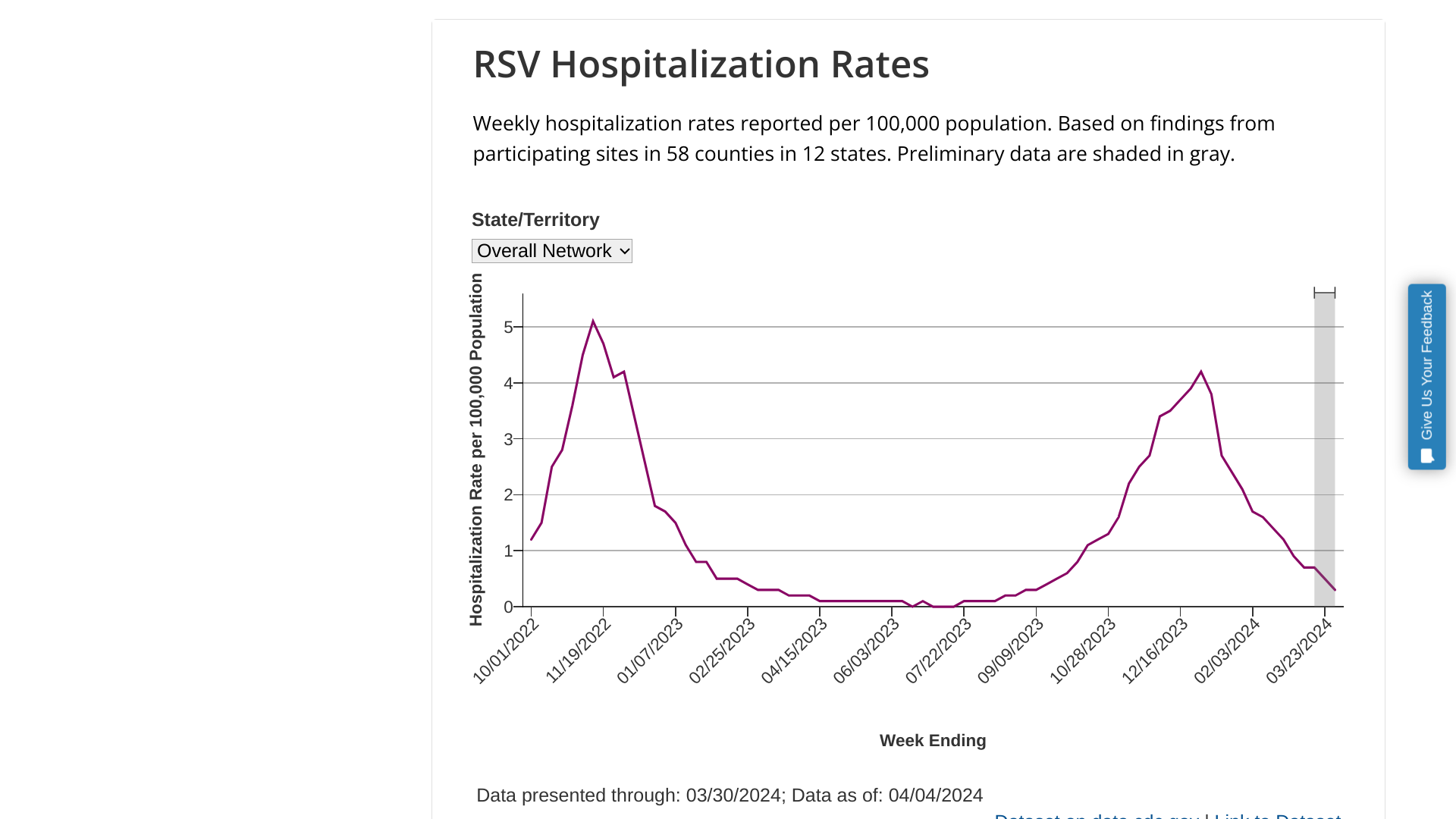
Pfizer Inc. today reported encouraging top-line data regarding the safety and immunogenicity of its ABRYSVO® vaccine from an ongoing Phase 3 clinical trial.
The study (NCT05842967) has been evaluating the effectiveness of a single dose of the vaccine compared to a placebo in adults between the ages of 18 and 59 who are at risk of developing severe lower respiratory tract disease associated with respiratory syncytial virus (RSV).
On April 8, 2024, Pfizer confirmed the MONeT study achieved its co-primary immunogenicity endpoints and primary safety endpoint:
- Participants demonstrated RSV-A and RSV-B subgroup neutralizing responses non-inferior to the response seen in the Phase 3 RENOIR study of ABRYSVO in more than 34,000 adults aged 60 or older where vaccine efficacy was previously demonstrated.
- Participants also achieved at least a four-fold increase in serum neutralizing titers for RSV-A and RSV-B one month after receiving ABRYSVO compared to pre-vaccination.
- During the trial, ABRYSVO was well-tolerated, and safety findings were consistent with those from previous investigations of ABRYSVO in other populations.
Pfizer stated it intends to submit these data to regulatory agencies and request expansion of the age group from the current indication to 18 years of age and older.
This is an essential study since no RSV vaccines were approved for adults in this age group during the 2023-2024 RSV season.
“These encouraging results provide evidence that ABRYSVO can help protect adults with increased risk against RSV-associated illness,” said Annaliesa Anderson, Ph.D., Senior Vice President and Head, Vaccine Research and Development, Pfizer, in a press release.
“We are excited to address a significant unmet need, pending regulatory authority approval, as ABRYSVO has the potential to become the first and only RSV vaccine for adults 18 years and older.”
As of April 5, 2024, RSV vaccines have been approved in Canada, Europe, Japan, the United States, and the United Kingdom.
The U.S. CDC estimates the percentage of adults 60+ vaccinated this season was 23.6%. And RSVVaxView reported that the overall RSV vaccination rate among pregnant women was about 18%.
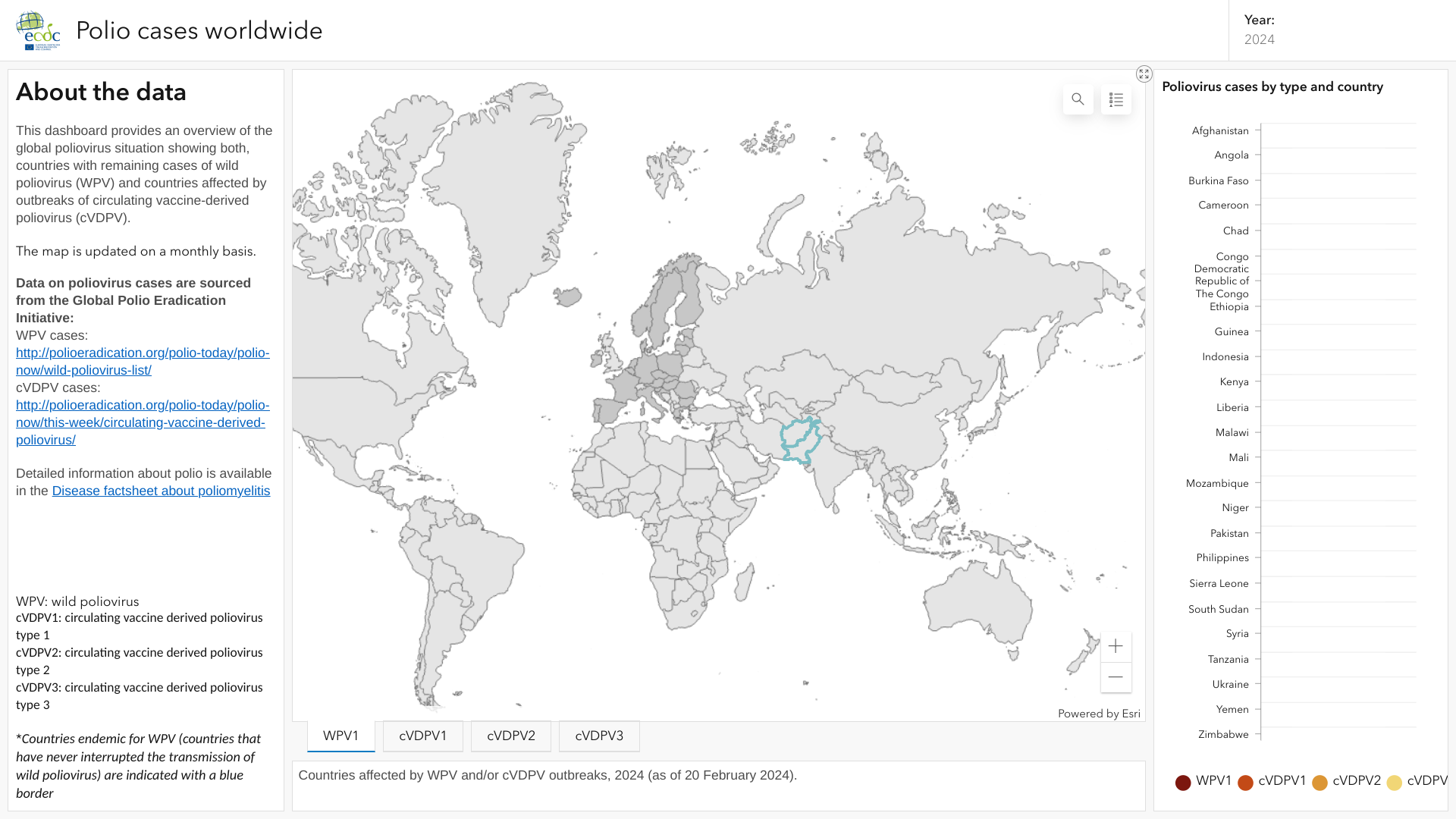
The World Health Organization (WHO) announced on April 8, 2024, during the 38th meeting of the IHR Emergency Committee for Polio, that the spread of the poliovirus remained a Public Health Emergency of International Concern (PHEIC).
The WHO committee also recommended extending the PHEIC for three more months, effective March 28, 2024, to reduce poliovirus outbreaks.
Previously, the European Centre for Disease Prevention and Control (ECDC) published an interactive map in February 2024 highlighting countries that continue confirming polio or poliovirus cases in 2024.
The ECDC says poliomyelitis, or polio, is a vaccine-preventable systemic viral infection. Historically, it has been a major cause of mortality, acute paralysis, and lifelong disabilities.
However, large-scale immunization programs have eliminated polio from most areas worldwide.
All health agencies recommend that international travelers be fully vaccinated before visiting countries report polio outbreaks.
Both inactivated and oral polio vaccines are available at clinics and pharmacies globally.
Furthermore, the U.S. CDC maintained its Global Polio Alert—Level 2, Travel Health Notice, regarding polio outbreaks and poliovirus detections in 31 countries.