Search API
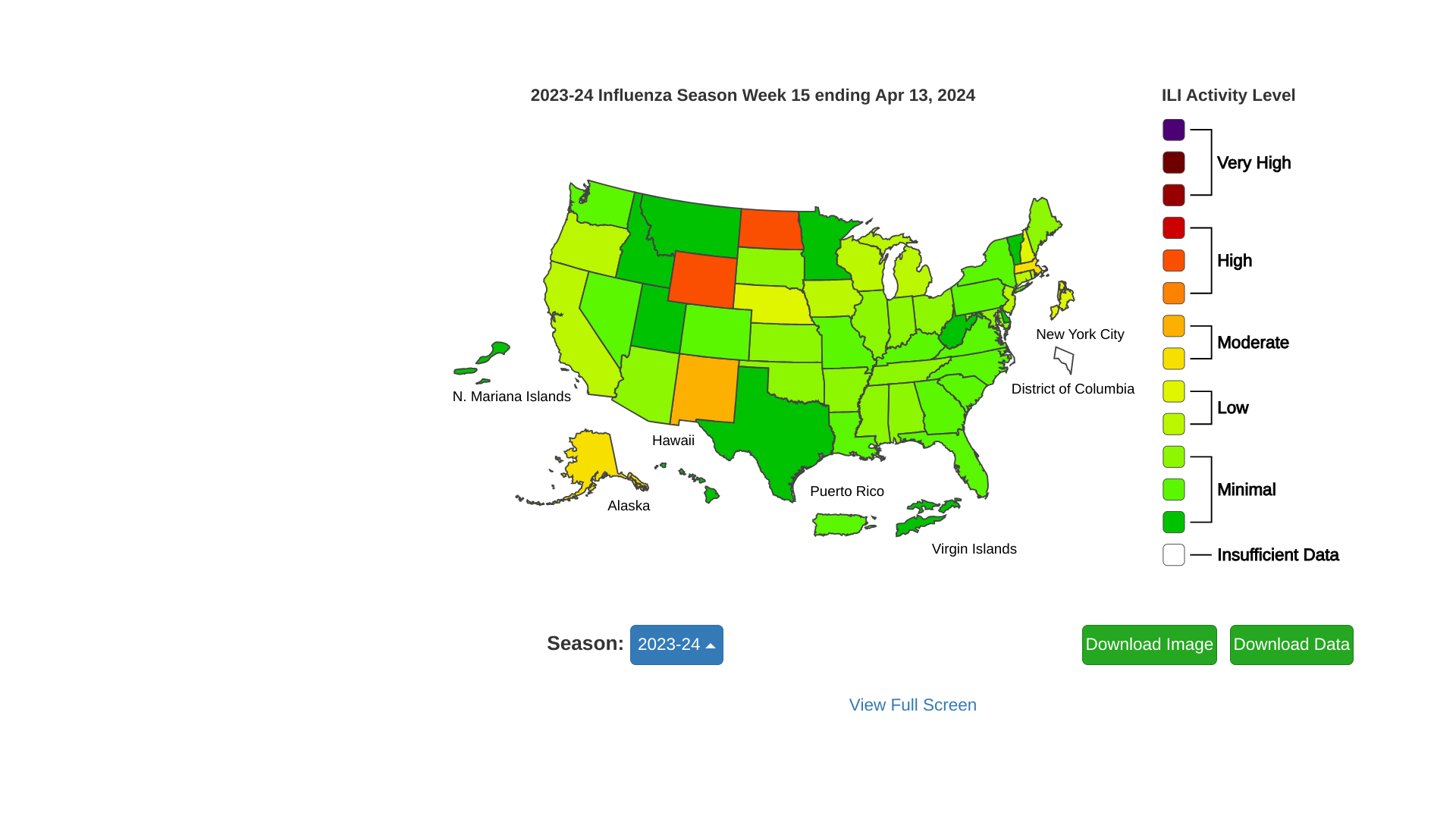
The U.S. CDC FluView report for week #15 stated seasonal influenza activity continues to decline in most areas of the United States.
Nationally, the number of weekly flu hospital admissions has been decreasing since January 2024.
Unfortunately, four influenza-associated pediatric deaths occurring during the 2023-2024 season were reported to the CDC last week, bringing the flu season total to 142 pediatric deaths.
As of April 19, 2024, the CDC recommends that everyone six months and older get an annual flu vaccine as long as influenza viruses spread.
Flu shots (egg, cell, nasal-based) can still provide benefits this season.
According to the recent Nationally Notifiable Infectious Diseases and Conditions weekly report, the number of mpox cases in the United States has more than doubled compared to Week #15 in 2023.
As of April 13, 2024, 750 mpox cases had been reported, compared to 336 cases at the same time last year.
The U.S. CDC highlights New York City (151), California (72), and Texas (55) as mpox case leaders.
The U.S. Health and Human Services (HHS) was initially charged with coordinating the federal response to the mpox outbreak. According to the General Accountability report issued on April 18, 2024, HHS is recommended to adopt a coordinated, department-wide program that incorporates input from external stakeholders to identify and resolve challenges.
In the United States, Bavarian Nordic's JYNNEOS® vaccine was initially offered to healthcare staff in Boston on May 24, 2022.
Since then, over 1.2 million (1-dose: 38.8% and 2-dose: 24.3%) JYNNEOS doses have been administered in U.S. Jurisdictions.
As of April 2024, JYNNEOS remains the only FDA-approved non-replicating smallpox and mpox vaccine for military and non-military use and has recently become commercially available at U.S. pharmacies.

The WHO Director-General convened the thirty-eighth meeting of the Emergency Committee on the international spread of poliovirus in late March 2024.
The Committee unanimously agreed that the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern and recommended the extension of Temporary Recommendations for a further three months until July 2024.
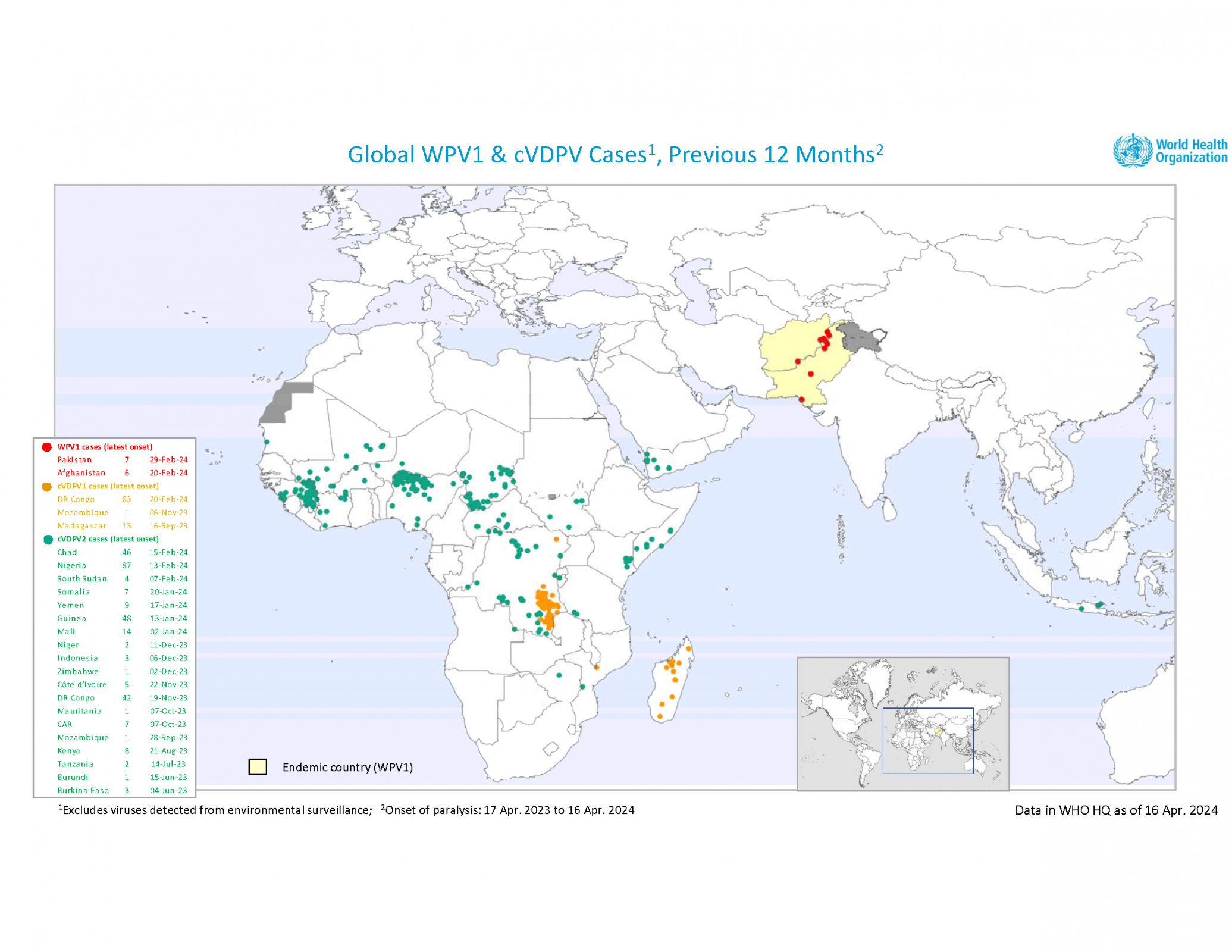
Regarding a weekly update, the Global Polio Eradication Initiative reported on April 17, 2024, that three African countries reported wild poliovirus (WPV1) and circulating vaccine-derived polioviruses (cVDPV) cases last week.
Chad reported one cVDPV2 case in Mandoul, making it the first in 2024. The number of 2023 cases remains 55.
The Democratic Republic of the Congo reported one cVDPV1 case in Haut-Katanga, the first this year. The number of polio cases in 2023 remains at 106, and 117 cases of cVDPV2 in 2023.
Nigeria confirmed one cVDPV2 case last week in Kebbi, the eighth this year. There have been 87 cases reported in 2023.
The U.S. CDC publishes travel advisories for countries reporting active polio cases and recommends fully vaccinating people before visiting these countries.
To understand polio vaccination options, including booster doses, the CDC suggests speaking with a travel vaccine advisor one month before traveling abroad.
Various media sources reported Merck would only supply 18.8 million HPV vaccine doses to Gavi-supported countries in 2024. Merck had previously promised to supply 29.6 million doses.
According to the Peoples Gazette, on April 19, 2024, Merck spokesman Patrick Ryan said the company "experienced a manufacturing disruption," requiring it to hold and check numerous doses manually.
Ryan disclosed that Burundi, Tajikistan, Mozambique, Sierra Leone, Ivory Coast, and Burkina Faso will not receive HPV vaccines in 2024.
By the end of 2022, 32 countries had successfully launched their HPV vaccine national program with Gavi support, fully immunizing more than 16.3 million girls since 2014.
GAVI says HPV vaccination is critical to reducing cervical cancer, especially in lower-income countries with a high disease burden and less developed cervical cancer screening and treatment programs.
On March 13, 2024, Merck announced plans to initiate clinical development of a new investigational multi-valent HPV vaccine designed to provide broader protection against multiple HPV types.
Separately, the company also plans to conduct clinical trials in both females and males to evaluate the efficacy and safety of a single-dose regimen of the GARDASIL®9 vaccine compared to the approved three-dose regimen.
"Evidence continues to emerge showing the importance of GARDASIL and GARDASIL 9 to public health," said Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, in a press release.
"These significant investments build upon our leadership and, importantly, provide the opportunity to further impact the global burden of certain HPV-related cancers and diseases."
The latest addition to Merck's pipeline employs the company's proprietary virus-like particle (VLP) technology to incorporate additional VLPs for expanded HPV-type coverage.
This includes several types known to have a greater impact on African and Asian populations and individuals of African and Asian descent. First-in-human phase 1 clinical studies are scheduled to start in the fourth quarter of 2024.
In the U.S., HPV vaccines remain available at clinics and pharmacies in 2024.

For many years, oral vaccines have proven to be the quickest intervention for preventing, limiting, and controlling cholera outbreaks.
However, the supply of these vaccines was at an all-time low in 2024, especially in 23 countries, including Comoros, the Democratic Republic of the Congo, Ethiopia, Mozambique, Somalia, Zambia, and Zimbabwe.
To help relieve this inventory shortage, the World Health Organization (WHO) issued a new oral cholera vaccine (OCV) prequalification.
On April 12, 2024, EuBiologicals Co., Ltd.'s inactivated oral vaccine, Euvichol-S, which has similar efficacy to existing vaccines but a simplified formulation, was announced.
This authorization creates new opportunities to increase OCV production capacity rapidly.
"The new vaccine is the third product of the same family of cholera vaccines on our WHO prequalification list," said Dr Rogerio Gaspar, Director of the WHO Department for Regulation and Prequalification, in a press release on April 18, 2024.
The WHO's OCV prequalification list already includes Euvichol, Euvichol-Plus, Vaxchora®, Dukoral®, and Shanchol™.
When visiting countries with cholera outbreaks in 2024, the U.S. Centers for Disease Control and Prevention recommends OCV vaccination one month before traveling.

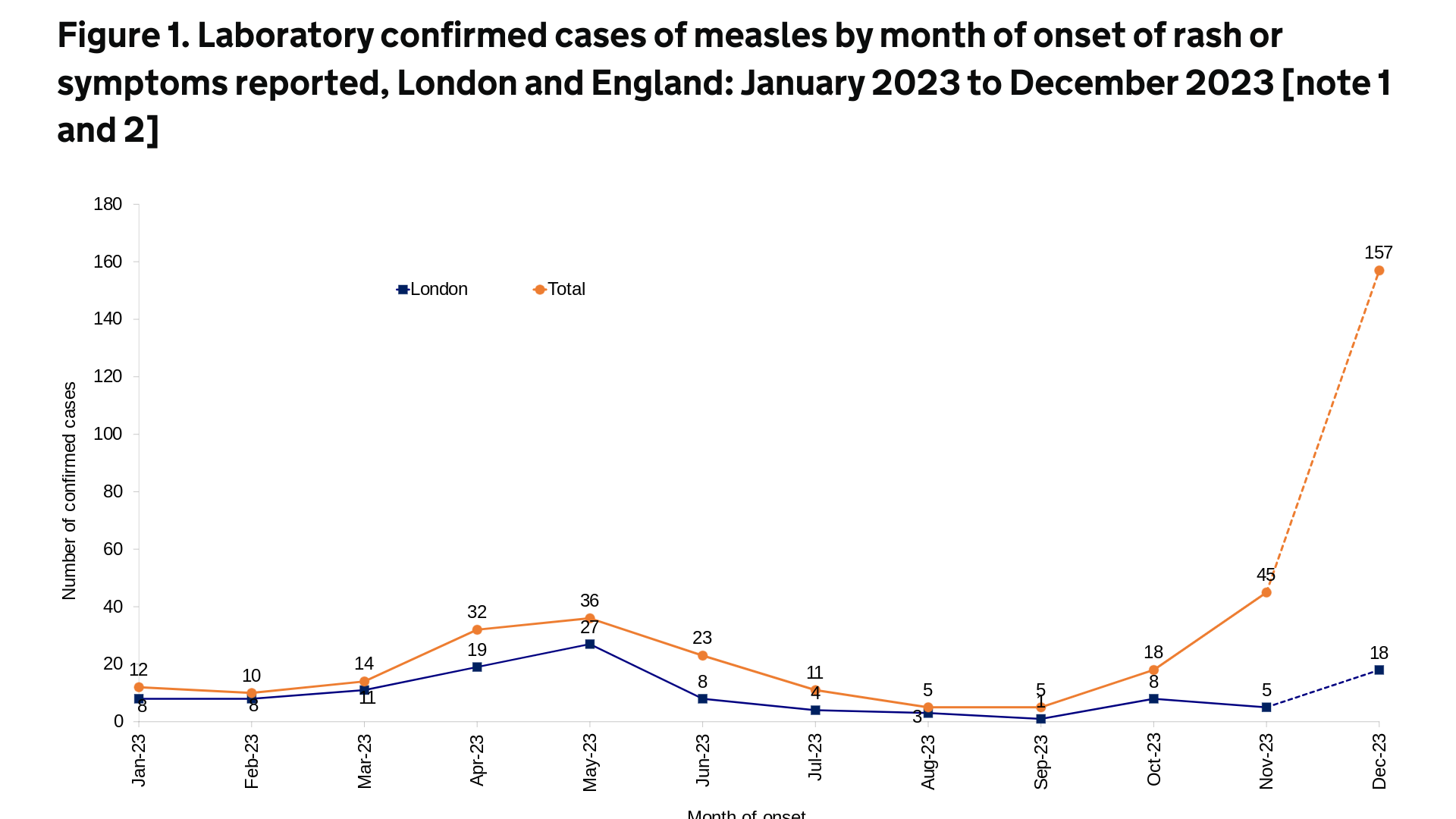
While measles outbreaks have been reported in dozens of countries in 2024, recent attention has focused on the cities of Chicago and London.
The U.K. Health Security Agency (UKHSA) published an updated epidemiological overview on April 18, 2024, stating that an additional 86 cases of measles were confirmed in England last week.
This data brings the total number of confirmed cases since October 2023 to 1,109.
About 39% of the U.K.'s measles cases (76 of 191) were in London during the last four weeks.
In a press release, Dr. Vanessa Saliba, UKHSA Consultant Epidemiologist, commented, "We know some communities in London have very low measles-mumps-rubella (MMR) vaccination rates. The MMR jab offers the best protection against measles."
The effective Priorx MMR vaccine is generally available at clinics and pharmacies in England. However, no measles-only vaccines are offered in England.
In the U.S., the Centers for Disease Control and Prevention (CDC) reported 121 measles cases in eighteen jurisdictions in 2024.
Most of these cases (61) have been reported by the Chicago Department of Public Health over the past two months.
The U.S. CDC republished a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice in March 2024, alerting international travelers of potential health risks and identifying measles outbreaks in 49 countries.
The CDC recommends speaking with a travel vaccine consultant one month before traveling abroad to any outbreak countries.