Search API
The Taiwan Centers for Disease Control (TCDC) recently announced new cases of mosquito-transmitted Dengue fever this year.
As of the end of September 2025, there have been 17 confirmed local cases of Dengue, with residents in Kaohsiung City (12 cases), Taoyuan City (4 cases), and Yilan County (1 case).
The TCDC reminds that although autumn has arrived, temperatures remain relatively high, making them suitable for the growth of mosquitoes that carry the disease. The public is advised to implement a "patrol, empty, sweep, and brush" approach to eliminate breeding sources to reduce mosquito density.
Additionally, the total number of imported cases this year is 181, the second-highest number in the same period over the past six years.
Most of the cases originated from Southeast Asian countries (92%), with Indonesia leading the way (56 cases), followed by Vietnam (42 cases), the Philippines (24 cases), and Thailand (20 cases).
Dengue cases have recently increased in neighboring Asian countries such as Bangladesh and China, with case numbers in Bangladesh, China, Vietnam, Sri Lanka, and Cambodia also exceeding the same period in 2024.
Taiwan medical institutions are also urged to heighten their vigilance and inquire more closely about patients' travel, occupational, contact, and social history.
They are also encouraged by the TCDC to use the dengue fever NS1 rapid screening test to assist in diagnosis and report cases early, enabling health authorities to implement preventive measures.
As of October 2025, fewer travel-related Dengue cases had been reported in the United States. However, the State of Florida continues to report both travel-related and locally acquired cases in 2025.
While Dengue is a vaccine-preventable disease, the current second-generation vaccine is unavailable in the United States.
The U.S. Food and Drug Administration recently conditionally approved Dectomax-CA1 (doramectin injection) injectable solution for the prevention and treatment of New World screwworm larval infestations, as well as the prevention of NWS reinfestation for 21 days.
Dectomax-CA1, sponsored by Zoetis, is based in Michigan and is conditionally approved for use in cattle only, not for human use.
"We understand the urgency with which America's farmers and ranchers are asking for tools to fight New World screwworm," said FDA Commissioner Marty Makary, M.D., M.P.H., in a press release on September 30, 2025.
"Today's conditional approval – the first in the U.S. for NWS – shows our dedication to rapidly advancing important animal medicines when they are needed most. We continue to work tirelessly to complete the review of other NWS products to protect multiple animal species in the U.S."
Dectomax-CA1 is eligible for conditional approval because it is intended to prevent and treat a serious or life-threatening disease in cattle, it addresses an unmet animal health need, and demonstrating the effectiveness of the drug would require complex or complicated studies.
Dectomax is already fully approved under a New Animal Drug Application for treatment and control of specific nematode and arthropod parasites in cattle and swine. NWS fly larvae burrow into the flesh of cattle, causing severe wounds and death if untreated.
Dectomax and Dectomax-CA1 contain the same active ingredient (doramectin injection) at the same dose. Because the original approval of Dectomax included adequate target animal safety studies, manufacturing information, and human food safety information, the FDA did not require new information to support those aspects for the conditional approval of Dectomax-CA1.
To reduce the risk of antiparasitic resistance and preserve the effectiveness of drugs against other parasites, producers and veterinarians are encouraged to use antiparasitic drugs like Dectomax-CA1 only when medically necessary, in accordance with the product labeling, and as part of a comprehensive parasite management strategy.
According to the U.S. CDC, NWS is typically a disease of livestock but can also affect humans with open wounds; it can also occur in other body cavities with mucus membranes (e.g., nasal passages).
There is no medication to treat NWS; prevention and prompt removal are key.
As of late September 2025, NWS infections had been detected in the Mexican state of Nuevo Leónles, than 100 miles from the Texas border.

The Nigeria Centre for Disease Control and Prevention (NCDC) reported that since January 2025 and mid-September, a total of 7,673 suspected Lassa fever cases and 166 related fatalities have been reported.
Cumulatively, as at week #37, the Case Fatality Rate was 18.5%.
Ninety percent of confirmed cases are from the Nigerian states of Ondo, Bauchi, Edo, Taraba, and Ebonyi.
According to the NCDC, Lassa fever is an acute viral illness likely present in West African countries. The Lassa virus is primarily transmitted to humans via contact with food or household items contaminated with rodent urine or faeces.
As of September 30, 2025, the U.S. CDC has issued Travel Health Notices for diphtheria, measles, and polio, but not Lassa Fever. While approved vaccines are available for the three diseases, there are no vaccines available for Lassa.
However, the International AIDS Vaccine Initiative has developed a Lassa fever vaccine candidate, which is currently being evaluated in a Phase IIa clinical trial in Ghana, Liberia, and Nigeria.
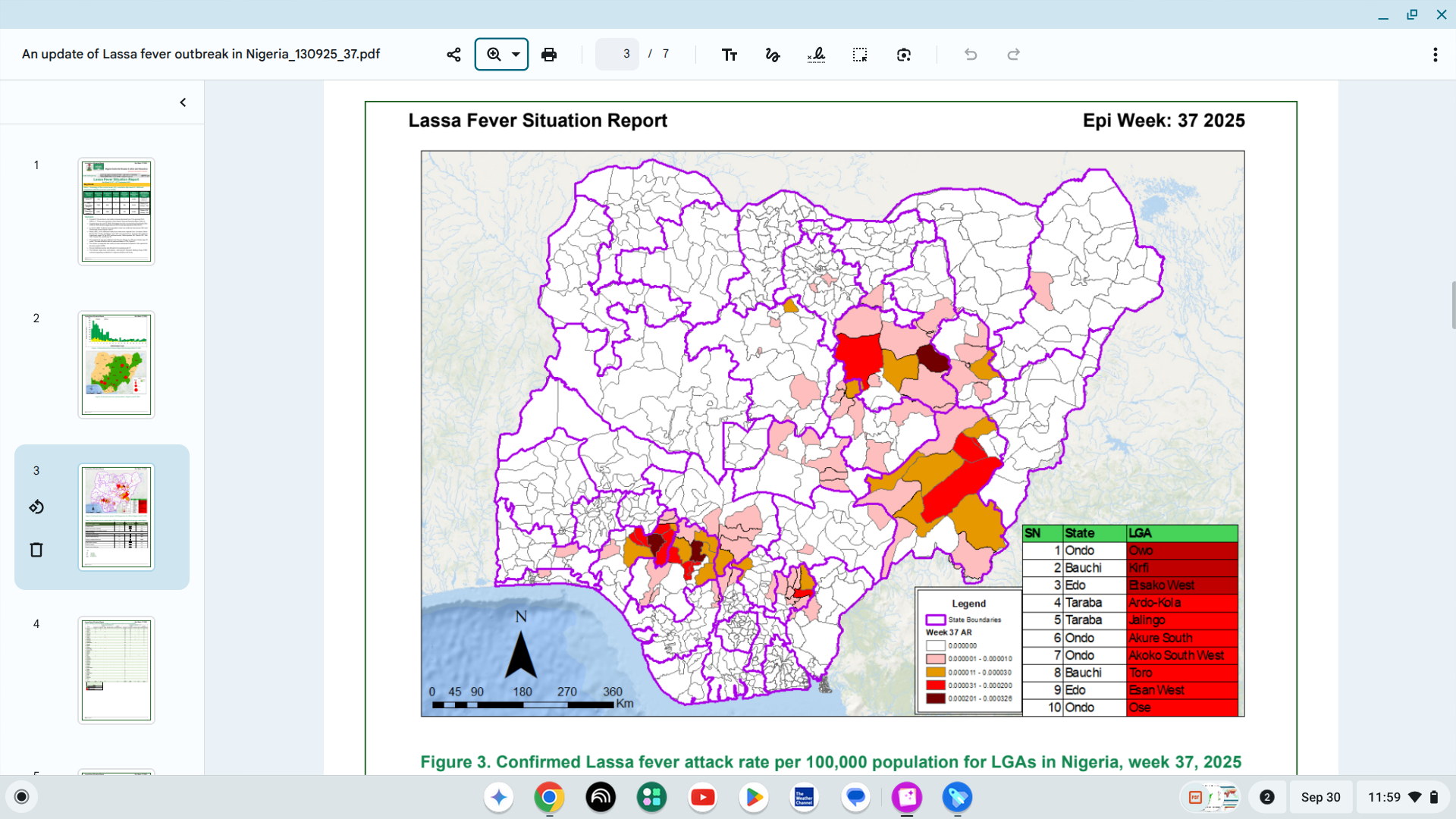
The US CDC recently published its Weekly US Influenza Surveillance Report: Key Updates for Week 38 of 2025, which indicates very few influenza cases have been reported this season.
As of late September, the percentage of respiratory specimens testing positive for the influenza virus in clinical laboratories was 0.4%. However, the 2025-2026 flu season generally accelerates in late Fall, peaking during the winter months.
This influenza test data indicates that people are seeing healthcare providers for respiratory illnesses referred to as influenza-like illnesses (fever plus cough or sore throat), not laboratory-confirmed influenza.
The CDC writes that this data may capture respiratory illness visits due to infection with any pathogen that can present with similar symptoms, including influenza, SARS-CoV-2, and RSV.
According to the World Health Organization (WHO), seasonal influenza vaccination can mitigate the impacts of annual outbreaks by preventing and mitigating the severity of infections.
As of mid-2025, 130 countries reported that seasonal flu shots were available in the public and/or private sector.
In the United States, as of September 30, 2-25, almost every pharmacy is currently offering influenza vaccinations.
Since rabies is a viral disease that is almost always fatal following the onset of clinical signs, immediate action is the best solution. 2025 marks the 19th World Rabies Day with the theme "Act Now: You, Me, Communities."
For the first time in its 19-year history, the theme does not include the word "rabies," highlighting the well-established nature of this movement, wrote the World Health Organization (WHO) on September 28, 2025.
"Whether you are an individual, part of an organization, or a decision-maker, the time to act is now."
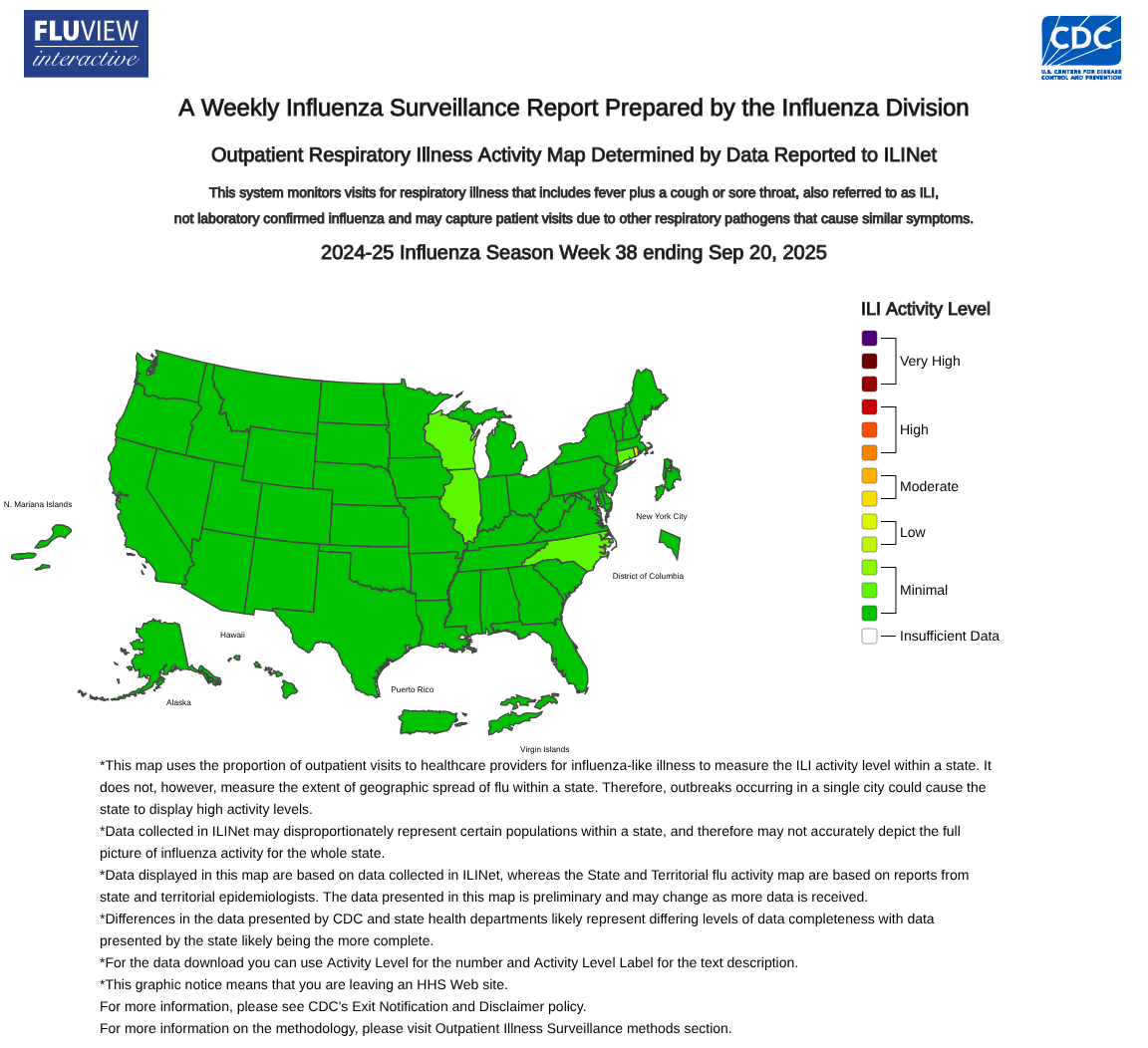
The WHO Global Health Observatory has been updated to include newly available rabies data, supporting data-driven policy and programming. This platform offers a clearer picture of global progress and remaining gaps in data and surveillance.
Globally, most rabies cases are attributed to dog bites, according to the WHO.
However, in the United States, most rabies infections in people are a byproduct of infected bat bites.
The U.S. CDC states that rabies post-exposure prophylaxis includes wound care, human rabies immune globulin, and a four-dose vaccine series.
As a preventive measure, Bavarian Nordic's RabAvert® rabies vaccine is often recommended by travel clinics and pharmacies in the U.S.
Since the beginning of 2025, over 317,000 Chikungunya virus disease cases and 135 related fatalities have been reported in 16 countries/territories in the Americas, Africa, Asia, and Europe.
To help reduce the impact of these mosquito-transmitted Chikungunya outbreaks, various countries have recently approved vaccines.
Regarding the long-term benefits, one vaccine manufacturer has presented very positive study results.
France-based Valneva SE today reported positive antibody persistence data four years after vaccination with a single dose of its chikungunya vaccine IXCHIQ®. The results confirm a strong and long-lasting antibody persistence across all age groups investigated.
Among the 254 healthy adults still followed in the clinical trial, 95% maintained neutralizing antibody titers well above the seroresponse threshold four years after the single-dose vaccination. The persistence of antibodies in older adults (aged 65+) was comparable to that in younger adults (18-64 years of age) in terms of geometric mean titers and seroresponse rates.
According to the trial protocol, antibody persistence is planned to be collected up to ten years after vaccination.
This study (VLA1553-303) has received funding support from the Coalition for Epidemic Preparedness Innovations and the European Union's Horizon Europe program. It has also collected long-term safety data up to two years, including Adverse Events of Special Interest from the preceding trial and any new-onset Serious Adverse Events.
No safety concerns were reported or identified.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on September 30, 2025, "We are very encouraged by these four-year data, which further reinforce IXCHIQ® 's unique profile and its ability to generate a robust, durable antibody response in both younger and older adults with just a single dose."
"Whether you are a traveler, live in an endemic area, or face an outbreak situation, the prospect of long-term protection from a mosquito-borne disease with a single vaccination is highly valuable, especially in Low- and Middle-Income Countries where vaccine access is often limited."
Chikungunya is a mosquito-borne viral disease spread by the bites of infected Aedes mosquitoes which causes fever, severe joint and muscle pain, headache, nausea, fatigue and rash.
Since the re-emergence of the virus, the European Centre for Disease Prevention and Control has reported that Chikungunya has now been identified in over 110 countries. Between 2013 and 2023, more than 3.7 million cases were reported in the Americas.

Global health leaders recently discussed the final steps needed to eradicate polio during an event titled "United to Finish the Job: High-Level Side Event on Polio Eradication," which was co-hosted by the Global Polio Eradication Initiative (GPEI).
Leadership from the GPEI emphasized the need for sustained funding to complete the eradication effort, highlighting the Kingdom of Saudi Arabia's pledge of $500 million to the GPEI. This funding, along with that from other critical donors, is enabling the partnership to reach hundreds of millions of children each year with lifesaving polio vaccines and ultimately end the transmission of the virus.
Dr Tedros Adhanom Ghebreyesus, WHO Director-General, commented in a press release on September 22, 2025, "Polio eradication is a shared responsibility."
"We can finish the job only through sustained collaboration and commitment from donors such as the Kingdom of Saudi Arabia, which has been an essential supporter through its critical political, social, technical, and financial contributions."
The focus was on the urgent need to end wild polio transmission in Afghanistan and Pakistan, while also addressing outbreaks of variant poliovirus in fragile settings across Africa and Asia.
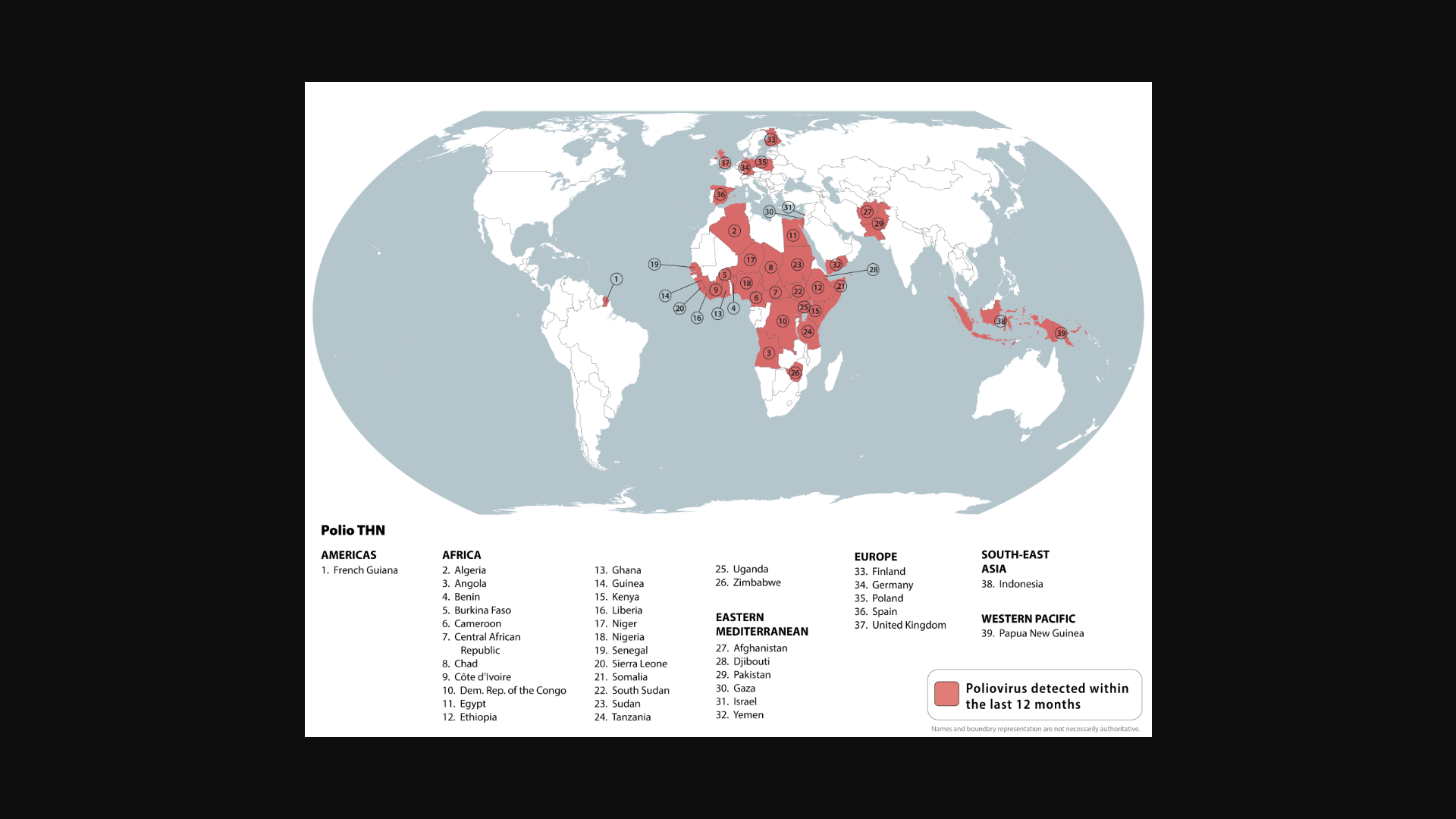
These countries and others were highlighted in the U.S. CDC's Global Polio Travel Health Notice, Level 2 - Practice Enhanced Precautions. The CDC identified 39 countries where poliovirus remains a health risk.
The CDC list includes the United Kingdom, which has previously reported detections of poliovirus in wastewater samples in London.
The CDC recommends that before any international travel, you ensure you are up to date on your polio vaccines. Adults who have previously completed the entire routine polio vaccine series may receive a single lifetime booster dose of polio vaccine.